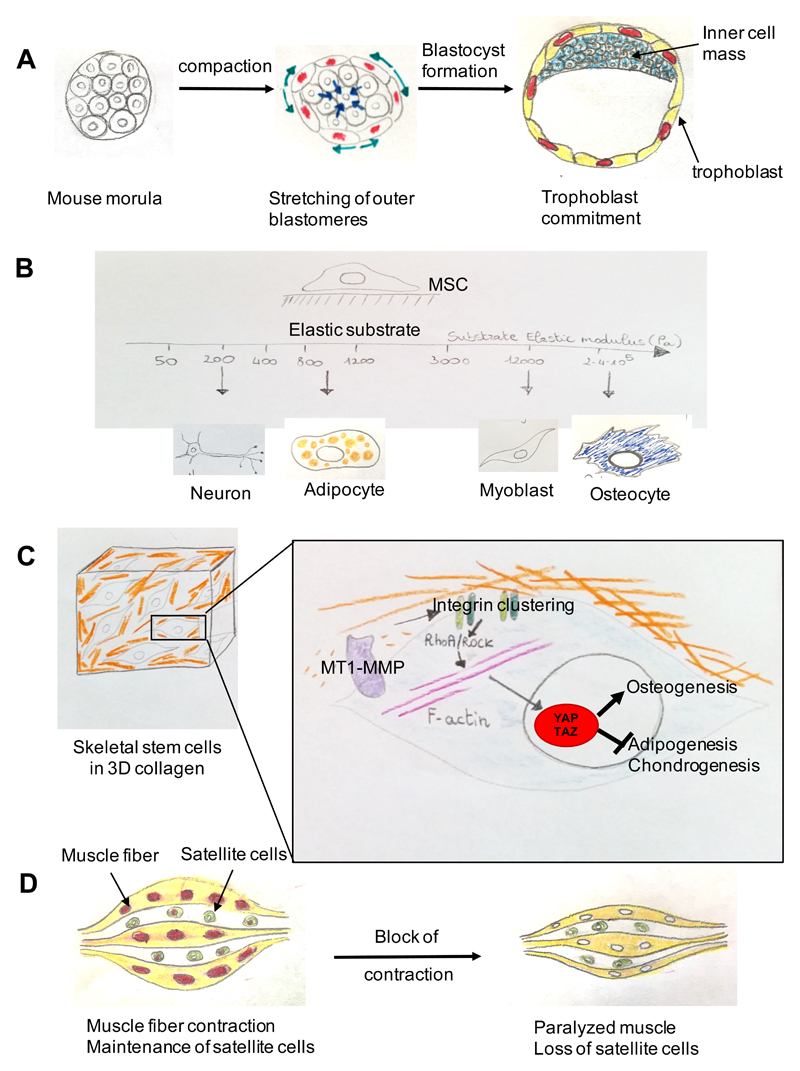
Figure 5. YAP and TAZ mechanotransduction in stem cell biology.
(a) Trophoblast commitment in the early mouse embryo involves a phase of stretching of the outer cells caused by compaction (inward pointing arrows) of the embryo. This stretching causes YAP and TAZ nuclear translocation in the outer cells and the acquisition of a trophoblast fate. Cells that do not activate YAP and TAZ become the cells of the embryo proper (the inner cell mass)69,70. (b) In vivo cells experience a range of different ECM elasticities in different tissues. By recapitulating these different stiffnesses in vitro, it was found that mesenchymal stem cells (MSCs) differentiate optimally into neurons, adipocytes, skeletal muscle cells or osteoblasts at specific elasticities that match the physiological ECM stiffness of their corresponding natural niche. (c) Control of skeletal stem cell lineage commitment mediated by ECM remodelling by membrane type 1-matrix metalloproteinase 1 (MT1-MMP). By affecting the ECM and thereby the cell shape of mesenchymal stem cells in a collagen-based 3D environment, the MT1-MMP promotes integrin clustering and the concomitant activation of the integrin–RhoA pathway, thereby triggering YAP and TAZ activation, which switches on a programme favouring osteogenic differentiation over alternative adipogenic and chondrogenic cell fates80. (d) YAP- and TAZ-dependent maintenance of satellite cells (muscle progenitors) by muscle contraction. In the chick embryo contraction of post-mitotic muscle fibres triggers YAP-dependent, expression of the Delta-like ligand JAG2, that activates NOTCH in muscle progenitor cells and prevents their differentiation. Blocking embryonic muscle contraction (muscle paralysis) inhibits YAP and transcription of JAG2 in myofibres, which causes a shift towards satellite cell differentiation. Enhanced differentiation eventually depletes the pool of muscle progenitor cells and interferes with regenerative potential of the tissue87. Similarly, expression of Delta-like ligands, such as DLL1 and JAG2, are also downstream of YAP and TAZ in epidermal stem cells43.