Table 1. Participant demographics (n = 1,146).
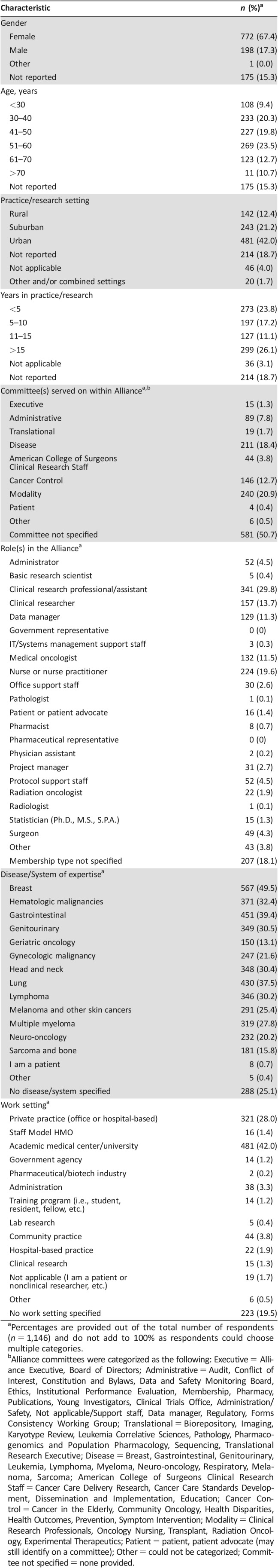
Percentages are provided out of the total number of respondents (n = 1,146) and do not add to 100% as respondents could choose multiple categories.
Alliance committees were categorized as the following: Executive = Alliance Executive, Board of Directors; Administrative = Audit, Conflict of Interest, Constitution and Bylaws, Data and Safety Monitoring Board, Ethics, Institutional Performance Evaluation, Membership, Pharmacy, Publications, Young Investigators, Clinical Trials Office, Administration/Safety, Not applicable/Support staff, Data manager, Regulatory, Forms Consistency Working Group; Translational = Biorepository, Imaging, Karyotype Review, Leukemia Correlative Sciences, Pathology, Pharmacogenomics and Population Pharmacology, Sequencing, Translational Research Executive; Disease = Breast, Gastrointestinal, Genitourinary, Leukemia, Lymphoma, Myeloma, Neuro‐oncology, Respiratory, Melanoma, Sarcoma; American College of Surgeons Clinical Research Staff = Cancer Care Delivery Research, Cancer Care Standards Development, Dissemination and Implementation, Education; Cancer Control = Cancer in the Elderly, Community Oncology, Health Disparities, Health Outcomes, Prevention, Symptom Intervention; Modality = Clinical Research Professionals, Oncology Nursing, Transplant, Radiation Oncology, Experimental Therapeutics; Patient = patient, patient advocate (may still identify on a committee); Other = could not be categorized; Committee not specified = none provided.
