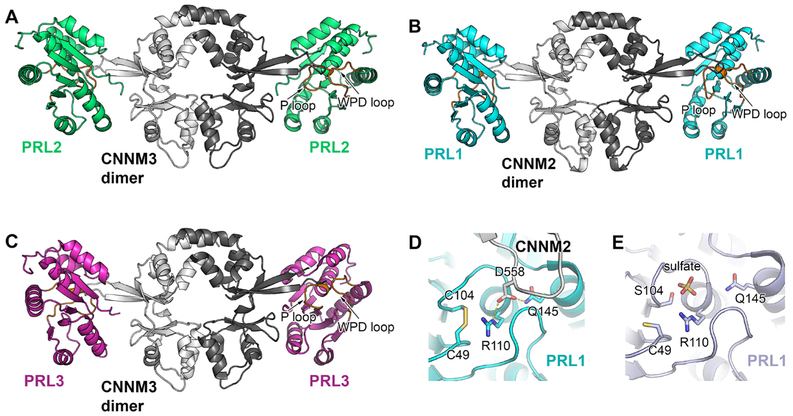
Fig. 4.
Structure of PRL-CNNM complex. A) Crystal structure of PRL-2 in complex with the CNNM3 CBS-pair domain (Protein Data Bank code 5K22) reveals a tetramer, where CNNM3 forms a dimer and binds to a PRL-2 protein at each side of the dimer. The interaction happens between the extended loop of CNNM3 and active site of PRL-2.B) Crystal structure of PRL-1-CNNM2 (Protein Data Bank code 5MMZ) and C) PRL-3-CNNM3 complex (Protein Data Bank code 5TSR) show similar interaction. D) and E) Comparison of PRL-1 active site conformation in the complex with CNNM2 with the conformation binding to a sulfate group that resemble its phosphate reveals similar conformation re-arrangement.