Abstract
Prostate cancer (PCa) is one of the most common malignancies among males worldwide. Anti-silencing function 1B histone chaperone (ASF1B) has been reported to be involved in PCa. The present study aimed to investigate the role and molecular mechanism of ASF1B in PCa. Data of genes were obtained from The Cancer Genome Atlas data- base. The core gene was identified using the DAVID website. Cell viability and colony formation were detected using a cell counting kit-8 assay and crystal violet staining, respectively. Cell cycle distribution and apoptosis were assessed using flow cytometry analysis. The corresponding factors were analyzed by reverse transcription-quantitative polymerase chain reaction and western blotting. It was demonstrated that ASF1B was highly expressed in the PCa tissues and cells compared with the non-PCa tissues and cells, respectively. While siRNA-ASF1B significantly reduced the viability and colony formation, it promoted apoptosis, G1 phase cell cycle arrest of LNCap as well as C4-2 cells. siRNA-ASF1B was revealed to significantly reduce the level of B-cell lymphoma-2 and cyclin D1, and enhance the expression levels of p53, caspase-3 and Bcl-2 associated X protein. Furthermore, the phosphorylation levels of phosphatidylinositol 3 kinase (PI3K) and protein kinase B (Akt) were significantly decreased in the siRNA-ASF1B group compared with the mock group. In summary, the present study demonstrated that silencing of ASF1B suppressed the proliferation, and promoted apoptosis and cell cycle arrest of PCa cells. Inhibition of the PI3K/Akt signaling pathway was pertinent to the role of si-ASF1B. This phenomenon suggests that the downregulation of ASF1B may aid in inhibiting the progression of PCa.
Keywords: anti-silencing function 1B histone chaperone, prostate cancer, apoptosis, phosphatidylinositol 3 kinase, protein kinase B pathway
Introduction
Prostate cancer (PCa) is one of the most common malignancies among males worldwide (1,2). The incidence of PCa has continued to rise in developed countries in recent years (3-5). To the best of our knowledge, no clinical symptoms are observable in the early stage of the cancer, and therefore a majority of patients are diagnosed with PCa in its advanced stage. The primary treatment methods of PCa are surgery, radiotherapy, cryosurgery, chemotherapy and endocrinotherapy. However, the effects of these treatments are not satisfactory, with the 5-year survival rate of patients with distant metastasis in the United States remaining ~20% (4,6-8). Therefore, it is necessary to identify a safe and effective strategy for treating PCa.
Cancer is known to be a genotypic disease, and therefore the occurrence of cancer is associated with changes to gene expression (9-11). In recent years, researchers have investigated target genes of cancer, and suggest that targeting therapy may be used to slow down the progression of cancer (12-15). Several studies have demonstrated that various genes, including melanoma differentiation associated gene-9/syntenin, sirtuin 1 and lysine demethylase 1A, contribute to the growth, metastasis and differentiation of PCa (16-19). Thus, determining target genes associated with the progression of PCa is required.
The phosphatidylinositol 3 kinase (PI3K)/protein kinase B (Akt/PKB) signaling pathway is widely distributed in cells, and contributes to the regulation of cell growth, proliferation and differentiation (20,21). Furthermore, the PI3K/Akt signal transduction pathway serves an essential role in the occurrence, development, treatment and prognosis of malignant tumor (22,23). PI3K, a bridge molecule associated with extra- cellular signal and cellular responses, serves an essential role in regulating cell apoptosis. Akt is a type of serine/threonine protein kinase. When cells are stimulated by extracellular signals, PI3K activates Akt activity and Akt further activates its downstream factors (24-26).
The present study aimed to explore the genes associated with the survival time of patients with PCa. Anti-silencing function 1B histone chaperone (ASF1B) was selected as a potential molecular involved in PCa and role was investigated. Thus, data from healthy adjacent and PCa tissues were collected from the Cancer Genome Atlas (TCGA) database. The differential genes among cancer and healthy adjacent tissues were analyzed using the edgeR software package. The core gene was identified using the DAVID website.
Materials and methods
Selection of core gene and determination of gene function
The genes in 499 PCa and 52 adjacent healthy tissues from the TCGA database were analyzed using the edgeR software package (http://www.bioconductor.org/packages/release/bioc/ html/edgeR.html). Next, 156 genes identified to be significantly associated with the survival of the patients were screened. The selected 156 genes were analyzed using the DAVID available online database (https://david.ncifcrf.gov/). The functions of the top seven core genes on the cell were investigated using Gene Ontology (GO) analysis (http://www.geneontology.org/). ASF1B was selected for subsequent experiments.
Tissue source
Between June 2015 and September 2017, 37 samples of PCa tissues and healthy adjacent tissues (mean age, 65.5±7 years) were obtained from patients with PCa who were admitted to The First Affiliated Hospital of Xinxiang Medical University (Xinxiang, China). Patients were diagnosed with PCa by biopsy or pathology. Patients who also had other malignant tumors, coronary heart disease or diabetes were excluded. All patients signed informed consent for the use of their tissues in the present study. This research was approved by the Ethics Committee of The First Affiliated Hospital of Xinxiang Medical University.
Cell culture
Human prostatic hyperplasia epithelial cell line (BPH) and PCa cell lines (PC-3, DU145, LNCap, VcaP and C4-2) were purchased from Beijing Zeping Technology Co., Ltd. (Beijing, China). PC-3 is derived from a bone metastatic site, and LNCap is derived from a left supraclavicular lymph node metastatic site. As a subline of LNCap, C4-2 exhibits androgen- independent growth in association with skeletal metastasis. DU145 is derived from a brain metastatic site and VcaP is derived from a vertebra metastatic site. BPH cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and penicillin-streptomycin mixed solution. PC-3, DU145, LNCap, VcaP and C4-2 cells were cultured in RPMI-1640 (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) with 10% FBS and 100X penicillin-streptomycin mixed solution. All cells were maintained in a humidified incubator with 5% CO2 at 37°C.
RNA interference and transfection
The human anti-silencing function 1 (ASF1)B-target small interfering (si)RNA and unspecific scrambled siRNA vectors were designed and synthesized by Nanjing Kehao Biotechnology Co., Ltd. (Nanjing, China). LNCap and C4-2 cells were seeded at a density of 1×104 cells/well into 6-well plates. The cells were transfected with siRNA-ASF1B (1 µg) or siRNA-negative control vector (1 µg) using Hieff Trans™ Liposomal Transfection reagent (Shanghai Yusheng Biotechnology Co., Ltd., Shanghai, China) following the manufacturer's protocol. The cells were incubated at 37°C for 12 h. Next, the cells were collected and prepared for the subsequent experiments.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Tissues and cells of total RNA were extracted using RNA extraction kit (Promega Corporation, Madison, WI, USA). A total of 1 µg of RNA was transcribed to cDNA using TianScript cDNA Synthesis kit (Tiangen Biotech Co., Ltd., Beijing, China). Reaction conditions were as follows: 85°C for 5 min, and at 4°C for 5 min. SYBR® Premix Ex Taq™ II kit (Takara, Beijing, China) was used to amplify cDNA under reaction conditions as follows: Predegeneration at 92°C for 5 min; denaturation at 92°C for 15 sec; annealing at 58°C for 30 sec for 30 cycles; and extension at 72°C for 35 sec. GAPDH was used as the loading control. The 2−ΔΔCq method was applied to compare gene expression levels. The primers used are listed in Table I.
Table I.
Sequences of the primers.
| Primer name | Sequence (5'-3') | Product size (bp) |
|---|---|---|
| ASF1B | 231 | |
| Forward | GATCAGCTTCGAGTGCAGTG | |
| Reverse | TGGTAGGTGCAGGTGATGAG | |
| p53 | 246 | |
| Forward | GCCCCTCCTCAGCATCTTAT | |
| Reverse | AAAGCTGTTCCGTCCCAGTA | |
| Cyclin D1 | 210 | |
| Forward | CCCTCGGTGTCCTACTTCAA | |
| Reverse | CTTAGAGGCCACGAACATGC | |
| Bax | 208 | |
| Forward | AACATGGAGCTGCAGAGGAT | |
| Reverse | CCAATGTCCAGCCCATGATG | |
| Bcl-2 | 207 | |
| Forward | TTCTTTGAGTTCGGTGGGGT | |
| Reverse | CTTCAGAGACAGCCAGGAGA | |
| Caspase-3 | 220 | |
| Forward | TGAGCCATGGTGAAGAAGGA | |
| Reverse | TCGGCCTCCACTGGTATTTT | |
| GAPDH | 222 | |
| Forward | CCATCTTCCAGGAGCGAGAT | |
| Reverse | TGCTGATGATCTTGAGGCTG |
ASF1B, anti-silencing function 1B histone chaperone; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein.
Western blotting
The proteins of tissues and cells were collected using RIPA lysate buffer (Beijing Solarbio Science & Technology Co., Ltd.). Protein concentration was detected using a bicinchoninic acid assay. Proteins (25 µg/lane) were separated using 8% SDS-PAGE and were then transferred onto a polyvinylidene fluoride membrane. The membrane was blocked with 5% skimmed milk at room temperature 1.5 h. Next, the membrane was incubated with the primary antibodies (Table II) at 4°C for 24 h. The membrane was then bound to the donkey anti-mouse IgG (cat. no. ab150109; 1:6,000), goat anti-mouse IgG (cat. no. ab6785; 1:6,000) (both from Abcam, Cambridge, UK) and donkey anti-rabbit IgG (cat. no. NL004; 1:5,000; R&D Systems, Inc., Minneapolis, MN, USA) secondary antibodies at 37°C for 1 h. The signal was visualized using enhanced chemiluminescence (Sangon Biotech Co., Ltd., Shanghai, China). Quantity One 4.6.2 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to perform densitometry.
Table II.
Antibodies used in the present study.
| Antibody | Catalog number | Company | Host animal | Dilution |
|---|---|---|---|---|
| Anti-ASF1B | ab70126 | Abcama | Rabbit | 1:800 |
| Anti-p53 | AF1335 | R&D Systems, Inc.b | Goat | 1:600 |
| Anti-cyclin D1 | AF4196 | R&D Systems, Inc. | Goat | 1:500 |
| Anti-caspase-3 | MAB835 | R&D Systems, Inc. | Rabbit | 1:500 |
| Anti- Bcl-2 | 2872 | Cell Signaling Technology, Inc.c | Rabbit | 1:1,000 |
| Anti-Bax | 2772 | Cell Signaling Technology, Inc. | Rabbit | 1:1,000 |
| Anti-p-PI3K | ab182651 | Abcam | Rabbit | 1:600 |
| Anti-PI3K | ab191606 | Abcam | Rabbit | 1:800 |
| Anti-p-Akt | AF887 | R&D Systems, Inc. | Rabbit | 1:1,000 |
| Anti-Akt | MAB2055 | R&D Systems, Inc. | Mouse | 1:1,000 |
| Anti-GAPDH | 2275-PC-100 | R&D Systems, Inc. | Rabbit | 1:1,000 |
Cambridge, UK;
Minneapolis, MN, USA;
Danvers, MA, USA. ASF1B, anti-silencing function 1B histone chaperone; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; PI3K, phosphatidylinositol 3 kinase; Akt, Protein kinase B.
Cell counting kit-8 (CCK-8) analysis
Cell viability was examined using a CCK-8 kit (Beijing Solarbio Science & Technology Co., Ltd.) following the manufacturer's protocol. In brief, LNCap and C4-2 cells were plated in 96-well plates (1.5×103 cell/well) in an incubator for 24 h. Cells were exposed to PBS (control), unspecific scrambled siRNA plasmid (mock) and ASF1B-target siRNA (siRNA-ASF1B) for 12, 24 and 48 h. Next, CCK-8 solution was added to the cells and incubated for 4 h. Absorbance at 450 nm was recorded and evaluated using a microplate reader.
Flow cytometry analysis
Cell apoptosis was examined using an Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). LNCap and C4-2 cells were inoculated in 6-well plates (3×104 cells/well) in the incubator for 24 h. All cells were treated as aforementioned. Next, cells were digested by 0.25% trypsin for 2 min at room temperature. The trypsin was discarded and cells were re-suspended in Annexin V binding buffer. The Annexin V-FITC and PI were added to cells and incubated for 20 min in the dark at room temperature. BD FACSAria I/II flow cytometry was performed to measure cell apoptosis and analysis was performed using BD CellQuest Pro 3.3 software (both from BD Biosciences, San Jose, CA, USA).
Cell cycle analysis was performed using a PI dye kit (Beyotime Institute of Biotechnology, Shanghai, China) following the manufacturer's protocol
Briefly, LNCap and C4-2 cells were treated as aforementioned. The PI and RNAse mix was added to the cells in the dark at 37°C for 20 min. Cell cycle was analyzed using flow cytometry.
Colony formation analysis
The colony formation of cells was determined using crystal violet staining (Beijing Solarbio Science & Technology Co., Ltd.). Briefly, LNCap and C4-2 cells were inoculated in 6-well plates (3×104 cell/well) and incubated for 24 h. All cells were treated as aforementioned. Cells were then fixed with 100% methanol for 10 min at room temperature. Next, cells were stained by 0.1% crystal violet for 5 min at room temperature. Finally, the cell colonies were observed under an inverted microscope (IX73; Olympus Corporation, Tokyo, Japan; magnification, ×2.5). The number of the colonies containing >50 cells was counted to calculate the colony formation as follows: (Number of colonies/number of cells) ×100%.
Statistical analysis
IBM SPSS Statistics 20.0 software (IBM, Corp., Armonk, NY, USA) was used for statistical analysis. Data are presented as the mean ± standard deviation. One-way analysis of variance followed by Tukey's text was performed to compare the differences among groups. The differences between tumor and healthy adjacent tissues were calculated using a paired Student's t-test. Kaplan Meier survival analysis with a log-rank test was performed to compare the overall survival rate of patients grouped by high and low ASF1B expression. The association between the expression of ASF1B and the clinicopathological features of patients with PCa were assessed using the Chi-square test. P<0.05 was considered to indicate a statistically significant difference. The experiments were independently repeated at least three times.
Results
ASF1B is identified as the core gene
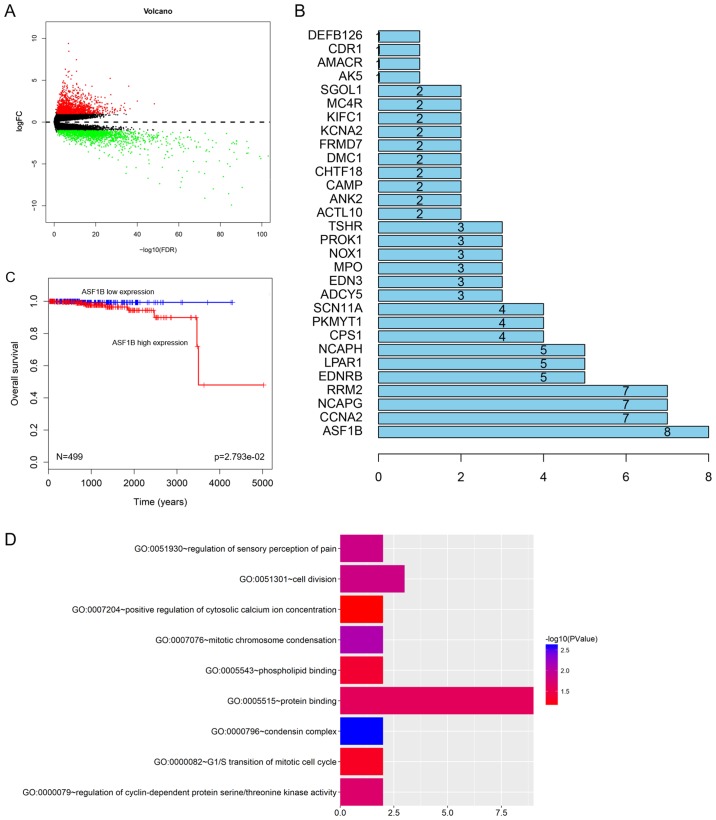
To identify the core gene, data from 52 healthy adjacent tissues and 499 PCa tissues were collected from the TCGA database. A total of 2,971 genes were investigated (Fig. 1A) and 156 genes were identified to be significantly associated with the survival rate of patients with PCa (Fig. 1B). A high expression of ASF1B was associated with the poor prognosis of patients with PCa (Fig. 1C). In addition, GO analysis demonstrated that seven core genes in PCa were significantly associated with cell cycle distribution and cell differentiation (Fig. 1D), the ASF1B gene was selected as the core gene in the present study. To determine the role of the expression of ASF1B in PCa, the pathological features of patients with PCa were analyzed. The data demonstrated the high ASF1B expression was significantly associated with tumor node metastasis (TNM) stage and metastasis; however, no significant association was identified between ASF1B expression and other pathological features (Table III; P>0.05).
Figure 1.
ASF1B is identified as the core gene. (A) The gene with differential expression in 52 healthy adjacent tissues and 499 prostate cancer tissues from the TCGA database was analyzed using the edgeR software package. (B) A total of 156 genes with differential expression were identified to be significantly associated with survival time through analysis using DAVID. The association with ASF1B was the most evident. (C) The overall survival curve of PCa patients with high or low ASF1B expression. (D) The function of ASF1B was investigated using GO analysis (DAVID website). ASF1B, anti-silencing function 1B histone chaperone; FC, fold change; FDR, false discovery rate; TCGA, The Cancer Genome Atlas; GO, Gene Ontology.
Table III.
Association between the expression levels of ASF1B and the clinicopathological features of patients with prostate cancer.
| Features | Number of patients | Low ASF1B expression (%) | High ASF1B expression (%) | P-value |
|---|---|---|---|---|
| Age (years) | 0.538 | |||
| <55 | 16 | 10 (47.6) | 6 (37.5) | |
| ≥55 | 21 | 11 (52.4) | 10 (62.5) | |
| PSA (ng/ml) | 0.942 | |||
| <4 | 15 | 8 (44.4) | 7 (36.8) | |
| ≥4 | 22 | 10 (55.6) | 12 (63.2) | |
| TNM stage | 0.012a | |||
| I/II | 12 | 8 (57.1) | 4 (17.4) | |
| III/IV | 25 | 6 (42.9) | 19 (82.6) | |
| Histological differentiation | 0.060 | |||
| Well | 3 | 2 (12.5) | 1 (4.7) | |
| Moderate | 12 | 8 (50.0) | 4 (19) | |
| Poor | 22 | 6 (37.5) | 16 (76.3) | |
| Metastasis | 0.010a | |||
| No | 23 | 15 (83.3) | 8 (42.1) | |
| Yes | 14 | 3 (16.7) | 11 (57.9) |
P<0.05, Chi-square test. ASF1B, anti-silencing function 1B histone chaperone; PSA, prostate-specific antigen; TNM, tumor node metastasis classification of malignant tumors.
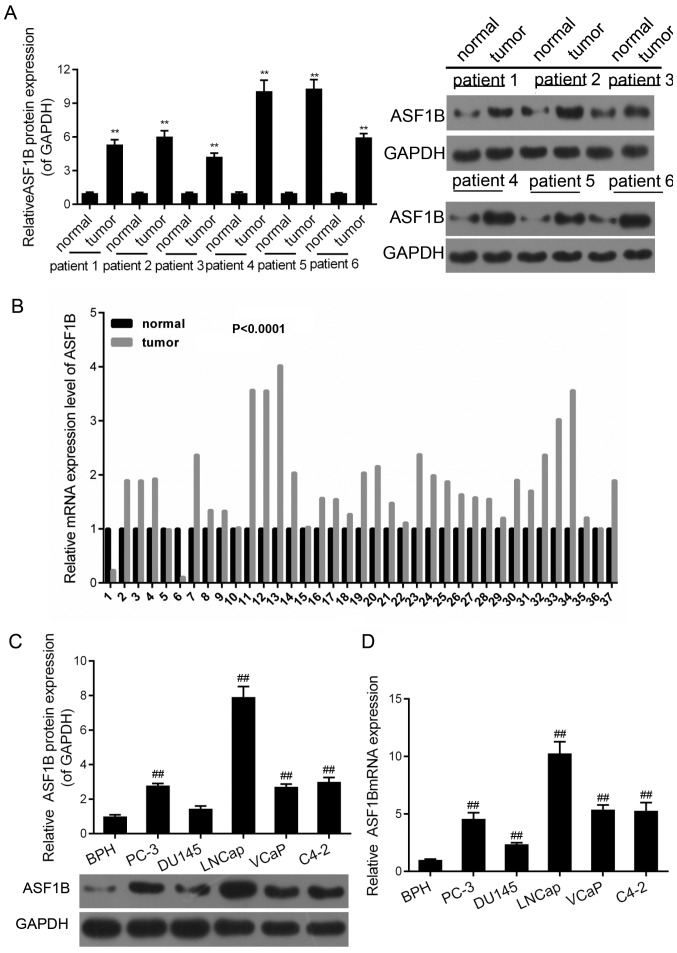
ASF1B is highly expressed in the PCa tissues and cells
RT-qPCR and western blotting were performed in order to examine the expression of ASF1B in the PCa and healthy adjacent tissues. It was demonstrated that the mRNA expression of ASF1B in cancer tissues were significantly higher compared with that in healthy adjacent tissues (Fig. 2A; P<0.01). A total of six patients from the cohort were randomly selected to measure the protein expression of ASF1B. The results revealed that there was at least a 2-fold increase in the protein expression of ASF1B in cancer tissues compared with that in the healthy adjacent tissues (Fig. 2B; P<0.01). Furthermore, the mRNA and protein levels of ASF1B in normal cells (BPH) were significantly lower compared with those in the PCa cells (PC-3, DU145, LNCap, VcaP and C4-2) (Fig. 2C and D; P<0.01). The expression levels of ASF1B in LNCap and C4-2 cells were the highest compared with those in other cell lines. Hence, LNCap and C4-2 cells were selected for subsequent studies.
Figure 2.
ASF1B is highly expressed in the prostate cancer tissues and cells. Between June 2015 and September 2017, 37 samples of prostate cancer tissues and adjacent tissues were obtained from patients with prostate cancer who were treated at the First Affiliated Hospital of Xinxiang Medical University. (A) Western blotting was performed to evaluate the protein expression of ASF1B in tissues (relative to the internal control, GAPDH). **P<0.01 vs. normal (healthy adjacent tissues). (B) RT-qPCR was used to detect the mRNA level of ASF1B in tissues. (C) The protein levels of ASF1B in BPH, PC-3, DU145, LNCap, VcaP and C4-2 were tested by western blotting (relative to the internal control, GAPDH). ##P<0.01 vs. BPH. (D) The mRNA levels of ASF1B in BPH, PC-3, DU145, LNCap, VcaP and C4-2 were assessed by RT-qPCR. GAPDH was presented as sample control. ##P<0.01 vs. BPH. ASF1B, anti-silencing function 1B histone chaperone; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
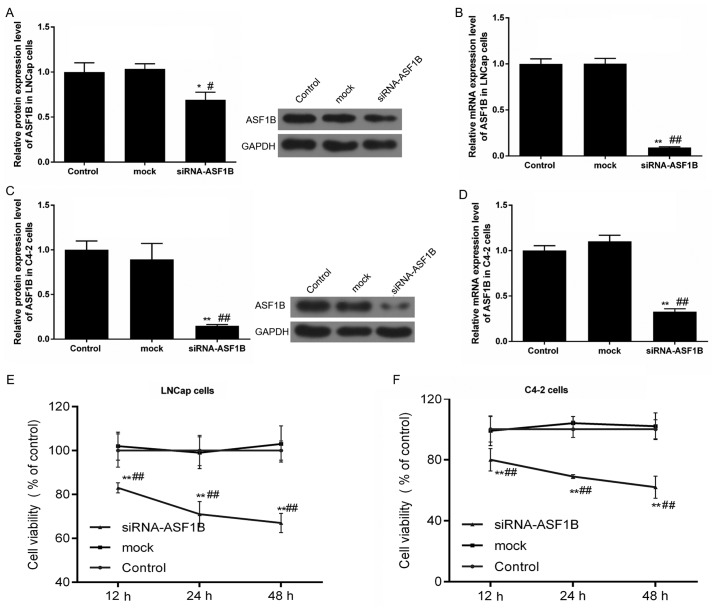
siASF1B decreases the viability of LNCap and C4-2 cells
The transfection efficiencies of the siRNA-ASF1B plasmid in LNCap and C4-2 cells were examined by RT-qPCR and western blotting. The results of the present study observed a significant decrease in ASF1B protein and mRNA expression (Fig. 3A and B; P<0.05) following the transfection of LNCap with the siRNA-ASF1B plasmid compared with the mock control group. Furthermore, the ASF1B protein and mRNA expression levels were reduced by siRNA-ASF1B transfection in C4-2 cells (Fig. 3C and D; P<0.05).
Figure 3.
siASF1B decreases the viability of LNCap and C4-2 cells. (A-D) LNCap and C4-2 cells were transfected with ASF1B-target siRNA (siRNA-ASF1B) and unspecific scrambled siRNA (mock) plasmid. The (A) protein and (B) mRNA levels of ASF1B were detected using western blotting (relative to the internal control, GAPDH) and RT-qPCR in LNCap cells. (C) The protein and (D) mRNA levels of ASF1B were detected using western blotting (relative to the internal control, GAPDH) and RT-qPCR in C4-2 cells. LNCap and C4-2 cells were treated with PBS (control), unspecific scrambled siRNA plasmid (mock) and ASF1B-target siRNA (siRNA-ASF1B) for 12, 24 and 48 h. A cell counting kit-8 was performed to examined the viabilities of LNCap (E) and C4-2 cells (F). *P<0.05; **P<0.01 vs. control; #P<0.05; ##P<0.01 vs. mock. ASF1B, anti-silencing function 1B histone chaperone; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; si, small inteferring RNA.
The viabilities of LNCap and C4-2 cells were examined using a CCK-8 assay. The CCK-8 data demonstrated that the viabilities significantly decreased in siRNA-ASF1B-transfected LNCap and C4-2 cells compared with the mock (Fig. 3E and F; P<0.01). Following transfection for 12 h, cell viability was significantly decreased with si-ASF1B. Thus, the si-ASF1B transfection duration was set as 12 h.
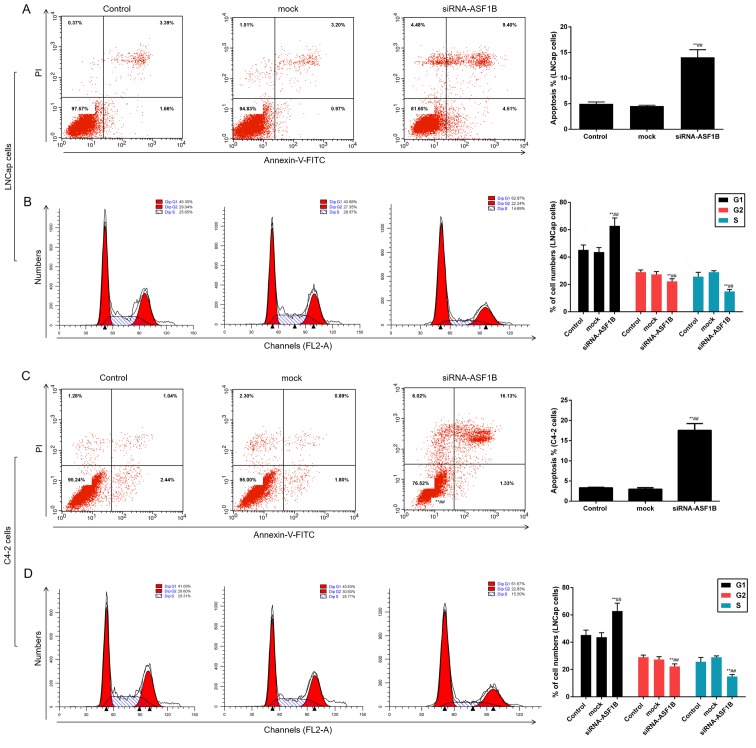
siASF1B induces apoptosis and arrests LNCap and C4-2 cells at the G1 phase
The data in the present study revealed that compared with the mock control, siRNA-ASF1B significantly enhanced the apoptotic and promoted the cell cycle arrest of LNCap cells (Fig. 4A and B; P<0.01). Additionally, when C4-2 cells were treated with siRNA-ASF1B, the apoptosis and the number of cells in G1 phase were significantly increased, whereas the number of cells in G2 and S phases was decreased significantly (Fig. 4C and D; P<0.01).
Figure 4.
siASF1B induces apoptosis and arrests LNCap and C4-2 cells at the G1 phase. Annexin V-FITC/PI apoptosis detection kit was performed to identify the apoptosis of LNCap and C4-2 cells. PI dye kit was performed to determine the cell cycle distribution. (A) Cell apoptosis and (B) cell cycle analysis of LNCap cells. (C) Cell apoptosis and (D) cell cycle analysis of C4-2 cells. **P<0.01 vs. control; ##P<0.01 vs. mock. ASF1B, anti-silencing function 1B histone chaperone; si, small inteferring RNA; PI, propidium iodide.
siASF1B inhibits the proliferation of LNCap and C4-2 cells
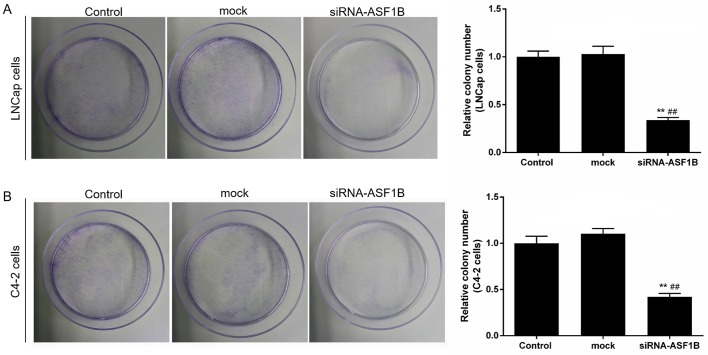
In order to identify the effect of siASF1B on the proliferation of LNCap and C4-2 cells, colony formation analysis was performed. The results revealed that the colony formation of LNCap and C4-2 cells was significantly attenuated by siRNA- ASF1B transfection compared with the mock group (Fig. 5; P<0.01).
Figure 5.
siASF1B inhibits the proliferation of LNCap and C4-2 cells. Crystal violet staining was used to examine the colony formation of (A) LNCap and (B) C4-2 cells Magnification, ×2.5. **P<0.01 vs. control; ##P<0.01 vs. mock. ASF1B, anti-silencing function 1B histone chaperone; si, small inteferring RNA.
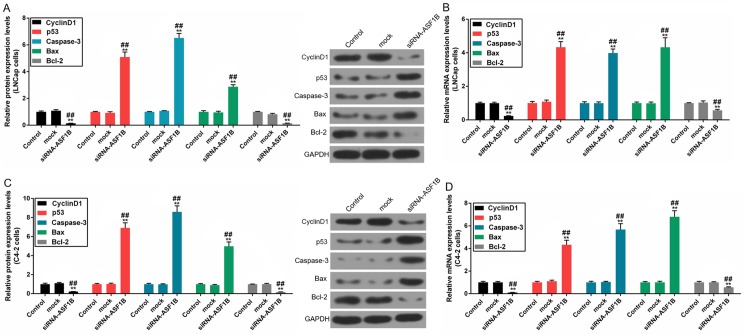
siASF1B regulates apoptosis-associated factors in LNCap and C4-2 cells
The results demonstrated that the protein and mRNA levels of cyclin D1 and Bcl-2 were downregulated; however, the protein and mRNA levels of p53, caspase-3 and Bax were upregulated in siRNA-ASF1B-transfected LNCap cells, compared with the mock group (Fig. 6A and B; P<0.01). In addition, the protein and mRNA expression levels of these factors in C4-2 cells were in accordance with those observed in LNCap cells (Fig. 6C and D; P<0.01).
Figure 6.
siASF1B regulates proliferation- and apoptosis-associated factors in LNCap and C4-2 cells. The protein and mRNA levels of p53, cyclin D1, caspase-3, Bax and Bcl-2 in LNCap and C4-2 cell were determined by western blotting (relative to the internal control, GAPDH) and reverse transcription- quantitative polymerase chain reaction. The (A) protein and (B) mRNA levels of p53, cyclin D1, caspase-3, Bax and Bcl-2 in LNCap cells. The (C) protein and (D) mRNA levels of p53, cyclin D1, caspase-3, Bax and Bcl-2 in.C4-2 cells. **P<0.01 vs. control; ##P<0.01 vs. mock. ASF1B, anti-silencing function 1B histone chaperone; si, small inteferring RNA; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2.
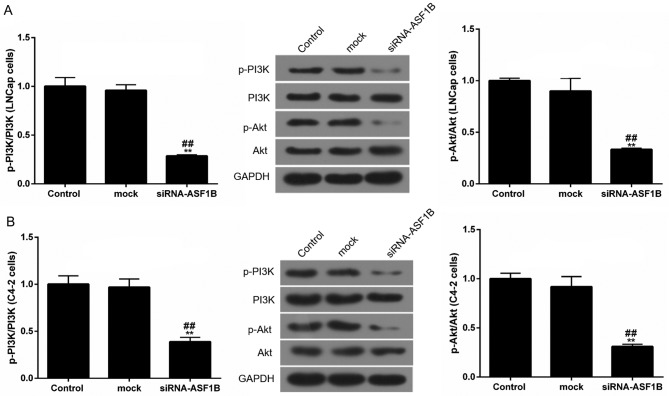
siASF1B suppresses the PI3K/Akt signaling pathway in LNCap and C4-2 cells
In order to further explore the possible mechanism of siASF1B in LNCap and in C4-2 cells, western blotting was performed to detect the protein expression levels of p-PI3K, PI3K, p-Akt and Akt. Western blotting data demonstrated that siRNA-ASF1B significantly reduced the phosphorylation levels of PI3K and Akt in LNCap and C4-2 cells by at least a third compared with the mock control group. However, the levels of PI3K and Akt remained stable among other groups. The proportions of p-PI3K/PI3K and p-Akt/Akt in siRNA-ASF1B were significantly reduced compared with those in the mock group (Fig. 7; P<0.01).
Figure 7.
siASF1B suppresses the PI3K/Akt pathway in LNCap and C4-2 cells. The protein levels of p-PI3K, PI3K, p-Akt and Akt in (A) LNCap and (B) C4-2 cells were examined using western blotting (relative to the internal control, GAPDH). **P<0.01 vs. control; ##P<0.01 vs. mock. ASF1B, anti-silencing function 1B histone chaperone; si, small inteferring RNA; PI3K, phosphatidylinositol 3 kinase; Akt, Protein kinase B; p, phosphorylated.
Discussion
In the present study, ASF1B was identified to be associated with cell cycle and cell differentiation in PCa. ASF1 was initially identified in yeast, and belongs to the histone H3-H4 chaperone protein. ASF1 regulates chromatin functions and has been demonstrated to contribute to tumorigenesis (27). ASF1 has two primary subtypes, which are ASF1A and ASF1B (28). Studies have reported that ASF1B is highly expressed in the human thymus and testicles (29). In addition, ASF1B has been reported to participate in the development of cervical and breast cancer (30,31). However, the role of ASF1B in PCa remains unclear. Therefore, the present study aimed to explore the function of ASF1B in PCa.
The results revealed that ASF1B was highly expressed in PCa tissues and cells compared with healthy tissues and cells, respectively. This result is similar to the high expression of ASF1B identified in breast cancer and cervical carcinoma (30,31). Furthermore, the results of the present study revealed that high expression of ASF1B was associated with a low overall survival rate, and that the expression of ASF1B was associated with TNM stage and metastasis.
A previous study has demonstrated that the overexpression of ASF1B markedly enhances the proliferation of breast cancer (30). Thus, we hypothesized that siASF1B may decrease the growth of PCa cells. The results also demonstrated that siRNA-ASF1B significantly decreased the viability and the colon formation of LNCap and C4-2 cells compared with control cells. Cell proliferation is associated with cell cycle distribution. Changes in proliferation participate in the regulation of cell cycle phases, including G1, G2 and S (32,33). In the present study, the data revealed that siRNA-ASF1B induced G1 phase cell cycle arrest. Consistently, researches have also confirmed previously that various anti-genes promote G1 phase arrest and inhibit the cyclin D1 expression in cancer cells (34-37). Similarly, the present results indicated that siRNA-ASF1B inhibited the expression levels of cyclin D1 in LNCap and C4-2 cells suggesting that ASF1B silencing promotes cell cycle arrest of PCa.
Apoptosis is another important process in the progression of tumors. Anticancer drugs and genes serve important roles in promoting the process of cancer apoptosis (38-40). Therefore, the effect of siASF1B on the apoptosis of PCa was analyzed. The results revealed that siRNA-ASF1B increased the apoptosis rate of LNCap and C4-2 cells. To further verify the inhibitory effect of siASF1B on apoptosis, factors associated with apoptosis were investigated. Bax and p53 are pro-apoptotic genes, and Bcl-2 is an anti-apoptotic gene (41,42). Additionally, studies demonstrated that the activity of caspase-3 is strengthened during the process of cell apoptosis (43-45). In the present study, siRNA-ASF1B significantly upregulated the levels of p53, cleaved caspase-3 and Bax. However, siRNA-ASF1B treatment significantly downregulated Bcl-2 expression. These results indicated that the knockdown of ASF1B accelerated the apoptosis of PCa cells by inhibiting Bcl-2 expression, and enhancing the expression levels of p53, caspase-3 and Bax. Consistently, ASF1B has been reported as a necessary factor for promoting cell proliferation in breast cancer (30). A previous study indicated that ASF1B may be a therapeutic target in cancer (46). Taken together, it was concluded that the downregulation of ASF1B exerted an antitumor effect on PCa by promoting cell apoptosis and cell cycle arrest.
The PI3K/Akt signaling pathway serves a role in cell growth and proliferation, and has been observed to be dysregulated in various cancer types (47). A previous study demonstrated that the PI3K/Akt signaling pathway is inactivated, whereas osthole promotes the apoptosis of esophageal squamous cell carcinoma (48). Furthermore, repression of the PI3K/Akt signaling pathway inhibits cell viability and may enhance the apoptosis of PCa (49), indicating the role of the PI3K/Akt signaling pathway during tumor progression. Thus, the expression of PI3K and Akt was determined in the present study in order to explore the role of the PI3K/Akt pathway in the antitumor effect of si-ASF1B. The data revealed that siRNA-ASF1B significantly reduced the phosphorylation levels of PI3K and Akt in LNCap and C4-2 cells, suggesting that the silencing of ASF1B decreased the activity of the signaling PI3K/Akt pathway in PCa. Thus, inhibition of the PI3K/Akt pathway may be a possible mechanism underlying the antitumor effect of si-ASF1B in PCa.
In conclusion, the results of the present study demonstrated that ASF1B was highly upregulated in PCa. ASF1B silencing significantly induced G1 phase arrest, promoted cell apoptosis, and inhibited colon formation in PCa cells. The inhibition of the PI3K/Akt signaling pathway may be a possible contributor to the production of antitumor effect of si-ASF1B. These results suggest that the depletion of ASF1B may inhibit the progression of PCa and may be a potential target in treating PCa.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Authors' contributions
GYH and XJZ wrote the manuscript. GYH, XJZ, PL, QFY, ZYL and QNY performed the experiments. GYH and XXW designed the study. XJZ, PL and XXW performed the data analysis. GYH, XJZ and XXW revised the manuscript. All authors reviewed the manuscript.
Ethics approval and consent to participate
All patients recruited to the present study provided written informed consent for the utilization of their tissue samples for clinical research. The project protocol was approved by the Ethics Committee of The First Affiliated Hospital of Xinxiang Medical University.
Patient consent for publication
Written informed consent was obtained from all participants for the publication of their data.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
Not applicable.
References
- 1.Pérez-Rambla C, Puchades-Carrasco L, García-Flores M, Rubio- Briones J, López-Guerrero JA, Pineda-Lucena A. Non-invasive urinary metabolomic profiling discriminates prostate cancer from benign prostatic hyperplasia. Metabolomics. 2017;13:52. doi: 10.1007/s11306-017-1194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell KJ, Del Mar C, Wright G, Dickinson J, Glasziou P. Prevalence of incidental prostate cancer: A systematic review of autopsy studies. Int J Cancer. 2015;137:1749–1757. doi: 10.1002/ijc.29538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman KE, Chen MH, Moran BJ, Braccioforte MH, Dosoretz D, Salenius S, Katin MJ, Ross R, D'Amico AV. Prostate cancer-specific mortality and the extent of therapy in healthy elderly men with high-risk prostate cancer. Cancer. 2010;116:2590–2595. doi: 10.1002/cncr.24974. [DOI] [PubMed] [Google Scholar]
- 4.Rim SH, Hall IJ, Massetti GM, Thomas CC, Li J, Richardson LC. Primary care providers' intended use of decision aids for prostate-specific antigen testing for prostate cancer screening. J Cancer Educ. 2018 doi: 10.1007/s13187-018-1353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin H, Park YH, Kim YG, Lee JY, Park J. Aqueous two-phase system to isolate extracellular vesicles from urine for prostate cancer diagnosis. PLoS One. 2018;13:e0194818. doi: 10.1371/journal.pone.0194818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li XX, Zhang YG, Wang D, Chen YF, Shan YH. Preventive effects of aspirin on cardiovascular complications in prostate cancer cases after endocrinotherapy. Asian Pac J Cancer Prev. 2015;16:4909–4913. doi: 10.7314/APJCP.2015.16.12.4909. [DOI] [PubMed] [Google Scholar]
- 7.Liu Q, Tong D, Liu G, Yi Y, Xu J, Yang X, Wang L, Zhang J, Ye J, Zhang Y, et al. A novel BRCA2 mutation in prostate cancer sensitive to combined radiotherapy and androgen deprivation therapy. Cancer Biol Ther. 2018;19:669–675. doi: 10.1080/15384047.2018.1451278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheng MX, Wan LL, Liu CM, Liu CX, Chen SS. Cytoreductive cryosurgery in patients with bone metastatic prostate cancer: A retrospective analysis. Kaohsiung J Med Sci. 2017;33:609–615. doi: 10.1016/j.kjms.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Augello G, Balasus D, Fusilli C, Mazza T, Emma MR, Giannitrapani L, Agliastro R, Cervello M, Montalto G. Association between MICA gene variants and the risk of hepatitis C virus-induced hepatocellular cancer in a sicilian population sample. OMICS. 2018;22:274–282. doi: 10.1089/omi.2017.0215. [DOI] [PubMed] [Google Scholar]
- 10.Concolino A, Olivo E, Tammè L, Fiumara CV, De Angelis MT, Quaresima B, Agosti V, Costanzo FS, Cuda G, Scumaci D. Proteomics analysis to assess the role of mitochondria in BRCA1- mediated breast tumorigenesis. Proteomes. 2018;6:6. doi: 10.3390/proteomes6020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y, Zhang C, Liang H, Hu S, Li P, Liu L, Duan X, Chen C, Zhang Y, Dai P. Dishevelled 1, a pivotal positive regulator of the Wnt signalling pathway, mediates 5-fluorouracil resistance in HepG2 cells. Artif Cells Nanomed Biotechnol. 2018 Mar 27; doi: 10.1080/21691401.2018.1453827. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Bohlen J, McLaughlin SL, Hazard-Jenkins H, Infante AM, Montgomery C, Davis M, Pistilli EE. Dysregulation of metabolic-associated pathways in muscle of breast cancer patients: Preclinical evaluation of interleukin-15targeting fatigue. J Cachexia Sarcopenia Muscle. 2018 doi: 10.1002/jcsm.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalimuthu S, Zhu L, Oh JM, Lee HW, Gangadaran P, Rajendran RL, Baek SH, Jeon YH, Jeong SY, Lee SW, et al. Regulated mesenchymal stem cells mediated colon cancer therapy assessed by reporter gene based optical imaging. Int J Mol Sci. 2018;19:19. doi: 10.3390/ijms19041002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu H, Schreiber SL, Stockwell BR. Targeting dependency on the GPX4 lipid peroxide repair pathway for cancer therapy. Biochemistry. 2018;57:2059–2060. doi: 10.1021/acs.biochem.8b00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tremblay-LeMay R, Rastgoo N, Chang H. Modulating PD-L1 expression in multiple myeloma: An alternative strategy to target the PD-1/PD-L1 pathway. J Hematol Oncol. 2018;11:46. doi: 10.1186/s13045-018-0589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das SK, Pradhan AK, Bhoopathi P, Talukdar S, Shen XN, Sarkar D, Emdad L, Fisher PB. The MDA-9/Syntenin/ IGF-1R/STAT3 axis directs prostate cancer invasion. Cancer Res. 2018;78:2852–2863. doi: 10.1158/0008-5472.CAN-17-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karbasforooshan H, Roohbakhsh A, Karimi G. SIRT1 and microRNAs: The role in breast, lung and prostate cancers. Exp Cell Res. 2018;367:1–6. doi: 10.1016/j.yexcr.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Li P, You S, Nguyen C, Wang Y, Kim J, Sirohi D, Ziembiec A, Luthringer D, Lin SC, Daskivich T, et al. Genes involved in prostate cancer progression determine MRI visibility. Theranostics. 2018;8:1752–1765. doi: 10.7150/thno.23180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sehrawat A, Gao L, Wang Y, Bankhead A, III, McWeeney SK, King CJ, Schwartzman J, Urrutia J, Bisson WH, Coleman DJ, et al. LSD1 activates a lethal prostate cancer gene network independently of its demethylase function. Proc Natl Acad Sci USA. 2018;115:E4179–E4188. doi: 10.1073/pnas.1719168115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brufsky AM, Dickler MN. Estrogen receptor-positive breast cancer: Exploiting signaling pathways implicated in endocrine resistance. Oncologist. 2018;23:528–539. doi: 10.1634/theoncologist.2017-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammadi A, Amooeian VG, Rashidi E. Dysfunction in brain-derived neurotrophic factor signaling pathway and susceptibility to schizophrenia, Parkinson's and Alzheimer's diseases. Curr Gene Ther. 2018;18:45–63. doi: 10.2174/1566523218666180302163029. [DOI] [PubMed] [Google Scholar]
- 22.Danielsen SA, Eide PW, Nesbakken A, Guren T, Leithe E, Lothe RA. Portrait of the PI3K/AKT pathway in colorectal cancer. Biochim Biophys Acta. 18552015:104–121. doi: 10.1016/j.bbcan.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Toren P, Zoubeidi A. Targeting the PI3K/Akt pathway in prostate cancer: Challenges and opportunities (Review) Int J Oncol. 2014;45:1793–1801. doi: 10.3892/ijo.2014.2601. [DOI] [PubMed] [Google Scholar]
- 24.Liu ST, Hui G, Mathis C, Chamie K, Pantuck AJ, Drakaki A. The current status and future role of the phosphoinositide 3 kinase/ AKT signaling pathway in urothelial cancer: An old pathway in the new immunotherapy era. Clin Genitourin Cancer. 2018;16:e269–e276. doi: 10.1016/j.clgc.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Pompura SL, Dominguez-Villar M. The PI3K/AKT signaling pathway in regulatory T-cell development, stability, and function. J Leukoc Biol. 2018;103:1065–1076. doi: 10.1002/JLB.2MIR0817-349R. [DOI] [PubMed] [Google Scholar]
- 26.Ramakrishnan V, Kumar S. PI3K/AKT/mTOR pathway in multiple myeloma: From basic biology to clinical promise. Leuk Lymphoma. 2018 Jan 11; doi: 10.1080/10428194.2017.1421760. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Seol JH, Song TY, Oh SE, Jo C, Choi A, Kim B, Park J, Hong S, Song I, Jung KY, et al. Identification of small molecules that inhibit the histone chaperone Asf1 and its chromatin function. BMB Rep. 2015;48:685–690. doi: 10.5483/BMBRep.2015.48.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Koning L, Corpet A, Haber JE, Almouzni G. Histone chaperones: An escort network regulating histone traffic. Nat Struct Mol Biol. 2007;14:997–1007. doi: 10.1038/nsmb1318. [DOI] [PubMed] [Google Scholar]
- 29.Umehara T, Horikoshi M. Transcription initiation factor IID-interactive histone chaperone CIA-II implicated in mammalian spermatogenesis. J Biol Chem. 2003;278:35660–35667. doi: 10.1074/jbc.M303549200. [DOI] [PubMed] [Google Scholar]
- 30.Corpet A, De Koning L, Toedling J, Savignoni A, Berger F, Lemaître C, O'Sullivan RJ, Karlseder J, Barillot E, Asselain B, et al. Asf1b, the necessary Asf1 isoform for proliferation, is predictive of outcome in breast cancer. EMBO J. 2011;30:480–493. doi: 10.1038/emboj.2010.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosty C, Sheffer M, Tsafrir D, Stransky N, Tsafrir I, Peter M, de Crémoux P, de La Rochefordière A, Salmon R, Dorval T, et al. Identification of a proliferation gene cluster associated with HPV E6/E7 expression level and viral DNA load in invasive cervical carcinoma. Oncogene. 2005;24:7094–7104. doi: 10.1038/sj.onc.1208854. [DOI] [PubMed] [Google Scholar]
- 32.Mens MMJ, Ghanbari M. Cell cycle regulation of stem cells by MicroRNAs. Stem Cell Rev. 2018;14:309–322. doi: 10.1007/s12015-018-9808-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uzquiano A, Gladwyn-Ng I, Nguyen L, Reiner O, Götz M, Matsuzaki F, Francis F. Cortical progenitor biology: Key features mediating proliferation versus differentiation. J Neurochem. 2018 Mar 23; doi: 10.1111/jnc.14338. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Jiang H, Zhu Y, Zhou Z, Xu J, Jin S, Xu K, Zhang H, Sun Q, Wang J, Xu J. PRMT5 promotes cell proliferation by inhibiting BTG2 expression via the ERK signaling pathway in hepatocellular carcinoma. Cancer Med. 2018;7:869–882. doi: 10.1002/cam4.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leem DG, Shin JS, Kim KT, Choi SY, Lee MH, Lee KT. Dammarane-type triterpene ginsenoside-Rg18 inhibits human non-small cell lung cancer A549 cell proliferation via G1 phase arrest. Oncol Lett. 2018;15:6043–6049. doi: 10.3892/ol.2018.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yue Y, Yuan Y, Li L, Fan J, Li C, Peng W, Ren G. Homeobox protein MSX1 inhibits the growth and metastasis of breast cancer cells and is frequently silenced by promoter methylation. Int J Mol Med. 2018;41:2986–2996. doi: 10.3892/ijmm.2018.3468. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Chen S, Wei C, Rankin GO, Rojanasakul Y, Ren N, Ye X, Chen YC. Dietary compound proanthocyanidins from Chinese bayberry (Myrica rubra Sieb. et Zucc.) leaves inhibit angiogenesis and regulate cell cycle of cisplatin-resistant ovarian cancer cells via targeting Akt pathway. J Funct Foods. 2018;40:573–581. doi: 10.1016/j.jff.2017.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin CL, Lee CH, Chen CM, Cheng CW, Chen PN, Ying TH, Hsieh YH. Protodioscin induces apoptosis through ROS-mediated endoplasmic reticulum stress via the JNK/p38 activation pathways in human cervical cancer cells. Cell Physiol Biochem. 2018;46:322–334. doi: 10.1159/000488433. [DOI] [PubMed] [Google Scholar]
- 39.Park CH, Han SE, Nam-Goong IS, Kim YI, Kim ES. Combined effects of Baicalein and Docetaxel on apoptosis in 8505c anaplastic thyroid cancer cells via downregulation of the ERK and Akt/mTOR pathways. Endocrinol Metab (Seoul) 2018;33:121–132. doi: 10.3803/EnM.2018.33.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torres RG, Casanova L, Carvalho J, Marcondes MC, Costa SS, Sola-Penna M, Zancan P. Ocimum basilicum but not Ocimum gratissimum present cytotoxic effects on human breast cancer cell line MCF-7, inducing apoptosis and triggering mTOR/Akt/ p70S6K pathway. J Bioenerg Biomembr. 2018;50:93–105. doi: 10.1007/s10863-018-9750-3. [DOI] [PubMed] [Google Scholar]
- 41.Beberok A, Wrześniok D, Rok J, Rzepka Z, Respondek M, Buszman E. Ciprofloxacin triggers the apoptosis of human triple- negative breast cancer MDA-MB-231 cells via the p53/Bax/ Bcl-2 signaling pathway. Int J Oncol: Mar. 8:2018. doi: 10.3892/ijo.2018.4310.. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 42.Mirakhor Samani S, Ezazi Bojnordi T, Zarghampour M, Merat S, Fouladi DF. Expression of p53, Bcl-2 and Bax in endometrial carcinoma, endometrial hyperplasia and normal endometrium: A histopathological study. J Obstet Gynaecol. 2018 Mar 21; doi: 10.1080/01443615.2018.1437717. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 43.Alotaibi MR, Hassan ZK, Al-Rejaie SS, Alshammari MA, Almutairi MM, Alhoshani AR, Alanazi WA, Hafez MM, Al-Shabanah OA. Characterization of apoptosis in a breast cancer cell line after IL-10 silencing. Asian Pac J Cancer Prev. 2018;19:777–783. doi: 10.22034/APJCP.2018.19.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu M, Li F, Wang W. Vitexin protects dopaminergic neurons in MPTP-induced Parkinson's disease through PI3K/Akt signaling pathway. Drug Des Devel Ther. 2018;12:565–573. doi: 10.2147/DDDT.S156920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai TC, Lai KH, Su JH, Wu YJ, Sheu JH. 7-Acetylsinumaximol B induces apoptosis and autophagy in human gastric carcinoma cells through mitochondria dysfunction and activation of the PERK/eIF2α/ATF4/CHOP signaling pathway. Mar Drugs. 2018;16:E104. doi: 10.3390/md16040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Almouzni G, Corpet A. Asf1b as a prognosis marker and therapeutic target in human cancer. 2013/0149320 A1. US. Filed May 31, 2011; issued June 13, 2013.
- 47.Davis WJ, Lehmann PZ, Li W. Nuclear PI3K signaling in cell growth and tumorigenesis. Front Cell Dev Biol. 2015;3:24. doi: 10.3389/fcell.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu X, Li Z, Li T, Long F, Lv Y, Liu L, Liu X, Zhan Q. Osthole inhibits the PI3K/AKT signaling pathway via activation of PTEN and induces cell cycle arrest and apoptosis in esophageal squamous cell carcinoma. Biomed Pharmacother. 2018;102:502–509. doi: 10.1016/j.biopha.2018.03.106. [DOI] [PubMed] [Google Scholar]
- 49.Wu Z, Zhu Q, Yin Y, Kang D, Cao R, Tian Q, Zhang Y, Lu S, Liu P. Traditional Chinese Medicine CFF-1 induced cell growth inhibition, autophagy, and apoptosis via inhibiting EGFR-related pathways in prostate cancer. Cancer Med. 2018;7:1546–1559. doi: 10.1002/cam4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.