Abstract
Background
Symptoms drive healthcare use among adults with atrial fibrillation, but limited data are available regarding which symptoms are most problematic and which patients are most at-risk. The purpose of this study was to: 1) identify clusters of patients with similar symptom profiles, 2) characterize the individuals within each cluster, and 3) determine whether specific symptom profiles are associated with healthcare utilization.
Methods
We conducted a cross-sectional secondary data analysis of 1,501 adults from the Vanderbilt Atrial Fibrillation Registry. Participants were recruited from Vanderbilt cardiology clinics, emergency department, and in-patient services. Subjects included in our analysis had clinically verified atrial fibrillation and a completed symptom survey. Symptom and healthcare utilization data were collected with the University of Toronto Atrial Fibrillation Severity Scale. Latent class regression analysis was used to identify symptom clusters, with clinical and demographic variables included as covariates. We used Poisson regression to examine the association between latent class membership and healthcare utilization.
Results
Participants were predominantly male (67%) with a mean age of 58.4 years (±11.9). Four latent classes were evident, including an Asymptomatic cluster (N=487, 38%), Highly Symptomatic cluster (N=142, 11%), With Activity cluster (N=326, 25%), and Mild Diffuse cluster (N=336, 26%). Highly Symptomatic membership was associated with the greatest rate of emergency department visits and hospitalizations (incident rate ratio 2.4, p<0.001).
Conclusions
Clinically meaningful atrial fibrillation symptom profiles were identified that were associated with increased rates of emergency department visits and hospitalizations.
Keywords: atrial fibrillation, emergency service, hospitalization, symptom cluster
Introduction
The perception of symptoms is a major factor in the decision to utilize healthcare services. Symptom perception refers to both the detection of symptoms and the interpretation of symptom meaning.1–4 For individuals with atrial fibrillation (AF), symptoms may be interpreted as relatively harmless, resulting in a ‘wait and see’ approach, or might be interpreted as life-threatening and prompt a decision to seek immediate medical attention.5, 6
Limited data are available regarding the relationship between AF symptoms and healthcare utilization outcomes.7 Between 1993 and 2004 emergency department (ED) visits for AF increased 88% in the United States (U.S.), with almost 64% of ED visits for AF resulting in a subsequent hospitalization.8 Globally, hospitalizations for AF have consistently increased over time, with the most dramatic increases occurring in adults age 65 and older.9 Two studies using the Outcomes Registry for Better Informed Treatment of AF found that symptoms rated as severe with the European Heart Rhythm Association (EHRA) classification system were a major predictor of hospitalizations.10, 11 However, the relationship between specific AF symptoms and healthcare utilization outcomes has not been reported. There are a wide range of symptoms experienced by individuals with AF including palpitations, chest pain, shortness of breath, dizziness, exercise intolerance, and fatigue.12 It is likely that distinct AF symptoms are interpreted differentially, resulting in disparate utilization of healthcare services depending on the specific symptoms present.
Symptom clusters are groups of 2 or more co-occurring symptoms that are related due to a shared mechanism, covariance, or effect on patient outcomes.13–16 Symptom clusters likely have a unique impact on healthcare utilization due to the multiplicative effect of co-occurring symptoms.2 There are two basic approaches to symptom cluster research: 1) clustering symptom variables and 2) clustering individuals into mutually exclusive groups with similar symptom profiles.15, 17 Prior work on AF symptom clusters used the approach of identifying clusters of symptom variables.18, 19 In this work, we use the approach of clustering AF patients in order to examine symptom profiles. Identifying symptom profiles associated with higher rates of healthcare utilization could improve our ability to risk stratify patients and provide individualized support to patients at highest risk for ED visits or hospitalizations. The purpose of this study was to: 1) identify clusters of patients with similar symptom profiles, 2) characterize the individuals within each cluster, and 3) determine whether specific symptom profiles are associated with healthcare utilization (AF-related hospitalizations and ED visits).
Methods
This was a cross-sectional secondary data analysis using de-identified data from the Vanderbilt AF Registry (VAFR), a single center clinical biorepository.20 VAFR prospectively enrolled AF patients and their family members between 2002 and 2015. Institutional Review Board (IRB) approval was obtained from Vanderbilt University for the registry, and from the University of Pennsylvania for this secondary data analysis. All VAFR participants provided written informed consent.
Study Population
Patients from Vanderbilt cardiology clinics, ED, and in-patient services were consecutively enrolled in VAFR between 2002 and 2015.20–22 Inclusion requirements were age 18 years or greater and AF or atrial flutter documented with an electrocardiogram, Holter monitor, rhythm strip, or event recorder. AF was defined as replacement of p-waves with rapid oscillations that varied in size, shape, and timing and were accompanied by irregular ventricular response when atrioventricular conduction was intact. Patients were excluded from VAFR if AF was only present within the first 90 days after cardiac surgery. Our study sample included the 1,501 adults from VAFR with a confirmed diagnosis of AF and a completed baseline symptom survey. We excluded individuals who had atrial flutter but not AF.
Measurement
Demographic and Clinical Characteristics
Participants in VAFR had a detailed sociodemographic, medical, and drug history taken upon enrollment. An investigator designed REDCap23 form was used to standardize data collection. A combination of patient-reported and medical record review data were collected by trained study personnel (registered nurses). AF duration was determined by subtracting the reported age of AF onset from age at consent. Paroxysmal AF was defined as lasting for at least 30 seconds and terminating spontaneously, persistent AF as lasting for 7 days or longer and requiring electrical or chemical cardioversion, and permanent AF as continuous AF for which a decision was made not to restore sinus rhythm. The echocardiogram or magnetic resonance imaging performed closest to the time of enrollment was used to record the ejection fraction for all participants.
Atrial Fibrillation Symptoms
All participants were asked to complete the University of Toronto AF Severity Scale (AFSS) upon enrollment in VAFR.20, 24, 25 The third section of the AFSS is a symptom subscale that provides information regarding the presence and frequency of 7 common AF symptoms (palpitations, shortness of breath at rest, shortness of breath with activity, exercise intolerance, dizziness, fatigue at rest, and chest pain).25 Specifically, participants are asked how often they have been bothered by (palpitations) in the past 4 weeks. Subjects respond separately for each symptom on a 6-point Likert scale ranging from none (1) to a great deal (6). Internal consistency and test-retest reliability for the symptom subscale have not been reported. However, the AFSS has been used to validate the Canadian Cardiovascular Society Severity in AF scale, a physician-rated measure of symptom severity, correlation between arrhythmia and symptoms, and functional impairment, with scores ranging 0–4: Between class 0 and 4, the AFSS symptom subscale scores increased more than four-fold, demonstrating the ability of this subscale to discern clinically meaningful differences in symptoms.26
Healthcare Utilization
The second section of the AFSS measures if and how often participants were cardioverted, hospitalized, visited the ED, and/or had specialist clinic appointments in the past 12 months related to their AF.24 Trained study nurses collected the AFSS either by telephone or during clinic visits, which potentially reduced self-report bias during the collection of these variables. We examined two healthcare utilization variables, hospitalizations and ED visits, because we believe they could be safely reduced with interventions aimed at improving symptom management and self-care. The AFSS healthcare utilization section has low but acceptable 3-month test-retest reliability (0.71) and internal consistency (Cronbach’s α, 0.67).27
Statistical Analysis
Clusters were identified using latent class analysis, a type of finite mixture model.28 Latent class analysis allows for the simultaneous examination of relationships between multiple variables, covariates, and outcomes.17, 28 The goal of latent class modeling is to probabilistically group every observation (patient) into unobserved (latent) classes based on patterns in their observed (manifest) variables; resulting in expectations regarding how that patient group responds to each observed variable in the model. A latent class regression model extends the basic latent class model and allows for the inclusion of covariates, which predict class membership.28
We conducted a latent class regression analysis in R 3.3.0 using the poLCA package.28–30 We used the 7 symptoms on the AFSS subscale as our manifest variables. We included 11 sociodemographic and clinical covariates in our latent class model that were known a priori to be associated with AF symptoms or symptom clusters.7, 12, 19, 26, 31–33 Covariates were retained in our final adjusted model if they were statistically significant (p < 0.05) or if their removal changed the strength of the association between covariates and the latent classes by more than 10%. PoLCA automatically listwise deletes observations with missing values on any covariate, therefore the initial sample size of 1,501 was reduced during analysis due to our inclusion of 11 sociodemographic and clinical covariates. The poLCA package estimates the model by maximizing the log-likelihood function. To ensure we found the global maximum of the log-likelihood, we conducted 100 random start repetitions of our final model. The ideal number of latent classes was determined using statistical fit indices (BIC, AIC, and Pearson’s χ2) combined with evaluation of the model for theoretical and clinical meaningfulness.28 We defined probabilities by class for each manifest variable as high (>0.5), moderate (0.2 and 0.5), or low (<0.2).
Our final step was to conduct two separate Poisson regression analyses that used measures of healthcare utilization as the dependent variables and latent class membership as the independent variable. The first regression analysis used AF-related ED visits as the dependent variable, and the second regression used AF-related hospitalizations. Because sociodemographic and clinical covariates that potentially affect symptoms and healthcare utilization (e.g. age, comorbidities) were entered into the latent class regression analysis as covariates, we did not use them again in these regression analyses. The healthcare utilization regression analyses and standard descriptive statistics were conducted in SAS version 9.4 (Cary, North Carolina). We considered p-values of <0.05 statistically significant.
Results
Sample Characteristics
Participants were predominantly male (67%) and ranged in age from 18.9 and 88.5 years, with a mean of 58.4 years (±11.9). The sample primarily consisted of individuals with paroxysmal (51.1%) and persistent (43.6%) AF. Our analytic sample size was reduced to 1,291 for our final model due to missing covariate values. We used the chi-square test to compare symptom severities of individuals that were and were not included in the analytic sample and found no statistically significant differences (p<0.05) in symptoms, gender, age, ejection fraction, and coronary artery disease. Individuals that were excluded had more permanent AF (14% vs. 5%), higher BMI (33 vs. 31), less anti-arrhythmic medication, more heart failure (19% vs. 14%), and less ablations (32% vs 55%). Sample characteristics are further detailed in Table 1.
Table 1.
Sample Characteristics
| Variable | N=1,291 |
|---|---|
| Sociodemographic Profile | |
| Age (years) | 58.4 (±11.9) |
| Male | 861 (67%) |
| Female | 430 (33%) |
| Caucasian | 1,239 (96%) |
| Clinical Profile | |
| AF Sub-Type | |
| Paroxysmal | 660 (51.1%) |
| Persistent | 563 (43.6%) |
| Permanent | 68 (5.3%) |
| AF Duration (years) | 4.4 (±5.5) |
| Body Mass Index | 30.9 (±6.4) |
| CHADS2 score | 1.1 (±1.0) |
| Left Atrial Diameter | 41.7 (±7.7) |
| Left Ventricular Ejection Fraction | 55.5 (±9.9) |
| Heart Failure | 177 (13.7%) |
| Hypertension | 800 (62.2%) |
| Coronary Artery Disease | 270 (20.9%) |
| History of AF Ablation | 713 (55.2%) |
| Anti-Arrhythmic Medication | 750 (58.1%) |
| Emergency Department Visits | 0.7 (±1.1) |
| Hospitalizations | 0.8 (±1.0) |
| Palpitation score | 2.7 (±1.7) |
| Short of Breath at Rest score | 2.1 (±1.5) |
| Short of Breath with Activity score | 3.0 (±1.7) |
| Exercise Intolerance score | 3.0 (±1.7) |
| Fatigue at Rest score | 2.2 (±1.5) |
| Dizziness score | 2.2 (±1.4) |
| Chest Pain score | 1.8 (±1.3) |
Values are expressed as N (%) or Mean (±SD). Symptom scores are derived from Likert scale ratings (range 1–6).
Latent Class Regression Analysis
Model Selection
Our analyses indicated that the optimal solution consisted of four classes. In the initial unadjusted model, fit statistics indicated that either a 3 or 4 class solution could be selected. Therefore, we analyzed both the 3 and 4 class solutions when adjusting for covariates in the model. Fit statistics for the final models did not precisely indicate which solution was most appropriate. The BIC treats Type I and II errors as equally undesirable, whereas the AIC treats Type II errors as the most undesirable. As a result, the AIC is more likely to indicate an overfit model;34 therefore, the model indicated by the BIC was selected. The symptom profiles of the various solutions were compared, and the 4-class solution also made the most theoretical and clinical sense, and as such was the optimal solution.
Latent Class Symptom Characteristics by Cluster Membership
The Asymptomatic cluster consisted of individuals with a high probability of responding “none” for every symptom (Figure 1). This cluster had the highest membership (N=487, 38%). The Highly Symptomatic cluster (N=142, 11%) is characterized by a high probability to answer “a great deal” for the symptoms of shortness of breath with activity and exercise intolerance, along with a moderate probability of experiencing a “fair amount” to “a great deal” of palpitations, shortness of breath at rest, and fatigue at rest. The With Activity cluster (N=326, 25%) is characterized by a moderate probability of experiencing most symptoms in the “a little” to “a lot” range. However, the most probable symptoms/symptom ratings in the With Activity cluster are shortness of breath with activity and exercise intolerance rated as “a fair amount” and “a lot”. The Mild Diffuse cluster (N=336, 26%) is characterized by a moderate probability of experiencing most of the symptoms “none”, “very little” or “a little”, and a high probability to report “none” for chest pain. In every class, chest pain was highly or moderately likely to be reported as “none”.
Figure 1.
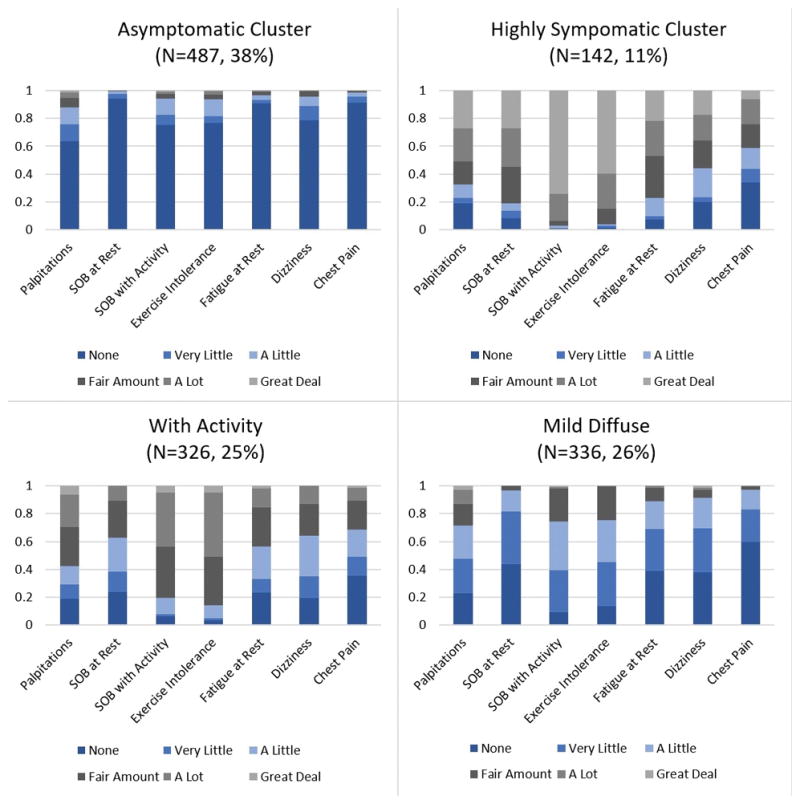
Graphical representation of manifest symptom variable probabilities by latent class membership. Scale represents probability between 0 and 1. SOB: shortness of breath.
Latent Class Covariates by Cluster Membership
Eleven sociodemographic and clinical variables that are known to influence AF symptoms (e.g. age, gender, comorbidities, AF type, ablation)12, 19, 26, 31–33 or were theoretically likely to influence AF symptoms (e.g. ejection fraction)7 were entered into our initial latent class model as covariates. Nine of these covariates were retained in the final model (only AF duration and ethnicity were removed), several of which had statistically significant variation from the reference (Asymptomatic) class (Table 2). Across each cluster, gender and history of ablation were the most consistently associated with cluster membership. Males made up the majority (77%) of the Asymptomatic cluster, whereas the male/female split was 50/50 in the Highly Symptomatic cluster, and close to equally divided in the With Activity cluster (54.6% male/45.4% female), despite the fact that the overall study population was 67% male and 33% female. The Asymptomatic cluster had the lowest percentage of patients with prior ablation. Individuals in the Highly Symptomatic cluster were less likely than those in the Asymptomatic cluster to have paroxysmal AF (32.4% versus 54.4%), and more likely to have persistent AF (61.3% versus 38%), a higher BMI (mean 33.8 versus 30), heart failure (33.8% versus 9.2%), and coronary disease (35.9% versus 17.3%). Individuals in the With Activity cluster were more likely than those in the Asymptomatic cluster to have a higher BMI (mean BMI 32 versus 30), be on anti-arrhythmic medication (69% versus 51%), and have coronary disease (28.2% versus 17.3%). The Mild Diffuse cluster was the only group that differed significantly by age, with a younger mean age of 56.9 compared to 58.6 for the Asymptomatic cluster (Table 3).
Table 2.
Latent Class Regression Model by Cluster Membership
| Cluster | β | SE | t |
|---|---|---|---|
| Highly Symptomatic Cluster vs Asymptomatic Cluster | |||
| Gender | 1.77786 | 0.27261 | 6.522† |
| Age | −0.01809 | 0.01321 | −1.370 |
| AF type | 0.53518 | 0.13616 | 3.931† |
| Ejection Fraction | −0.01776 | 0.01280 | −1.387 |
| Body Mass Index | 0.07231 | 0.01837 | 3.936† |
| Anti-arrhythmic | 0.37695 | 0.26663 | 1.414 |
| Heart Failure | 1.09543 | 0.33233 | 3.296* |
| Coronary Disease | 1.02034 | 0.30692 | 3.324* |
| Ablation | 1.01345 | 0.27692 | 3.660† |
| With Activity Cluster vs Asymptomatic Cluster | |||
| Gender | 1.32675 | 0.20351 | 6.519† |
| Age | −0.00975 | 0.00810 | −1.204 |
| AF type | 0.09330 | 0.10016 | 0.932 |
| Ejection Fraction | −0.00359 | 0.01041 | −0.345 |
| Body Mass Index | 0.04523 | 0.01489 | 3.038* |
| Anti-arrhythmic | 0.54034 | 0.18991 | 2.845* |
| Heart Failure | 0.39523 | 0.29892 | 1.322 |
| Coronary Disease | 0.81976 | 0.23296 | 3.519† |
| Ablation | 0.99310 | 0.18785 | 5.287† |
| Mild Diffuse Cluster vs Asymptomatic Cluster | |||
| Gender | 0.58792 | 0.21488 | 2.736* |
| Age | −0.01562 | 0.00785 | −1.989* |
| AF type | 0.20739 | 0.10087 | 2.056* |
| Ejection Fraction | −0.02074 | 0.01058 | −1.960 |
| Body Mass Index | 0.00117 | 0.01522 | 0.077 |
| Anti-arrhythmic | 0.07400 | 0.18210 | 0.406 |
| Heart Failure | 0.09176 | 0.32246 | 0.285 |
| Coronary Disease | −0.18319 | 0.28213 | −0.649 |
| Ablation | 0.81038 | 0.18568 | 4.364† |
Covariates that are significantly different from reference (Asymptomatic Cluster) are marked with an asterisk (*) for p<0.05, and with a dagger sign (†) for p<0.001.
Table 3.
Covariates by Cluster Membership
| Covariate | Asymptomatic Cluster (N=487) | Highly Symptomatic Cluster (N=142) | With Activity Cluster (N=326) | Mild Diffuse Cluster (N=336) |
|---|---|---|---|---|
| Gender | † | † | * | |
| Male | 377 (77.4%) | 71 (50%) | 178 (54.6%) | 235 (69.9%) |
| Female | 110 (22.6%) | 71 (50%) | 148 (45.4%) | 101 (30.1%) |
| Age | 58.6 (±12.4) | 59.8 (±10.5) | 59.1 (±12.2) | 56.9 (±11.6) * |
| AF Type | † | * | ||
| Paroxysmal | 265 (54.4%) | 46 (32.4%) | 181 (55.5%) | 168 (50%) |
| Persistent | 185 (38%) | 87 (61.3%) | 135 (41.4%) | 156 (46.4%) |
| Permanent | 37 (7.6%) | 9 (6.3%) | 10 (3.1%) | 12 (3.6%) |
| Ejection Fraction | 56.1 (±9) | 52.8 (±12.1) | 56.3 (±9.7) | 54.8 (±10) |
| Body Mass Index | 30 (±5.7) | 33.8 (±7.3) † | 32 (±6.9) * | 30 (±5.9) |
| Anti-arrhythmic | 251 (51.5%) | 91 (64.1%) | 225 (69%) * | 183 (54.5%) |
| Heart Failure | 45 (9.2%) | 48 (33.8%) * | 46 (14.1%) | 38 (11.3%) |
| Coronary Disease | 84 (17.3%) | 51 (35.9%) * | 92 (28.2%) † | 43 (12.8%) |
| Ablation | 202 (41.5%) | 89 (62.7%) † | 220 (67.5%) † | 202 (60.1%) † |
Values are expressed as N (%) or Mean (±SD);
p<0.05,
p<0.001.
Impact of Symptom Clusters on Healthcare Utilization
Emergency Department Utilization
Next, we conducted regression analysis with latent class membership as the independent variable and AF-related ED visits as the dependent variable. We used the Asymptomatic cluster as the reference. Membership in the Highly Symptomatic cluster was associated with nearly two and a half times the rate of AF-related ED visits as the Asymptomatic cluster (incident rate ratio (IRR) 2.37, p<0.001). The With Activity cluster also had an elevated rate of ED visits, with more than one and a half times the ED visits compared to the Asymptomatic cluster (IRR 1.7, p<0.001). The Mild Diffuse cluster was not associated with a significantly increased rate of ED visits compared to individuals in the Asymptomatic cluster (Table 4).
Table 4.
Unadjusted Incident Rate Ratios for AF-related ED Visits and Hospitalizations
| Emergency Visits | Hospitalizations | |||||
|---|---|---|---|---|---|---|
| Cluster | IRR | CI | p-value | IRR | CI | p-value |
| Asymptomatic | Reference | Reference | ||||
| Highly Symptomatic | 2.37 | [0.67, 1.06] | <0.001 | 2.36 | [0.67, 1.04] | <0.001 |
| With Activity | 1.7 | [0.37, 0.70] | <0.001 | 1.67 | [0.35, 0.68] | <0.001 |
| Mild Diffuse | 1.03 | [−0.16, 0.22] | 0.76 | 1.22 | [0.02, 0.37] | 0.03 |
IRR: incident rate ratio
Hospitalizations
Next, we conducted a regression with latent class membership as the independent variable and AF-related hospitalizations as the dependent variable. The Asymptomatic cluster was again used as the class of reference. Results are similar to those for ED visits. Membership in the Highly Symptomatic cluster was associated with nearly two and a half times the rate of AF-related hospitalizations compared to the Asymptomatic cluster (IRR 2.36, p<0.001). The With Activity cluster had over one and a half times the rate of hospitalizations as the Asymptomatic cluster (IRR 1.67, p<0.001). Mild Diffuse cluster membership was also associated with an increased the rate of hospitalizations, although to a lesser degree than the other clusters. Mild Diffuse cluster membership resulted in 1.22 times the rate of hospitalizations (p=0.03) compared to the Asymptomatic cluster (Table 4).
Discussion
We discovered 4 clusters of patients with unique symptom and covariate profiles. When we examined the 4 clusters for differences in rates of healthcare utilization, the results revealed that individuals in the Highly Symptomatic and With Activity clusters had statistically and clinically significant higher rates of both ED visits and hospitalizations compared to the Asymptomatic cluster. Individuals in the Highly Symptomatic cluster had the highest rates of healthcare utilization. Further, individuals in the Highly Symptomatic and With Activity clusters were fairly similar clinically in terms of the symptoms experienced and clinical covariates. The main clinical differences between the two clusters appear to be the higher probability of experiencing symptoms at rest (dyspnea and fatigue) and a greater proportion of patients with persistent AF, heart failure, and coronary artery disease in the Highly Symptomatic cluster.
The rate of healthcare utilization increased progressively starting with Asymptomatic, Mild Diffuse, With Activity, and Highly Symptomatic clusters. This could be interpreted as a reflection of overall symptom severity. These results align with prior research, which shows that a composite measure of physician-rated symptom severity, the EHRA severity scale, is a major predictor of hospitalization among adults with AF.10, 11 Our results expand on this prior knowledge by demonstrating that individuals with specific patient-reported symptom profiles have higher rates of ED visits and hospitalizations, with symptoms at rest (dyspnea and fatigue) being most probable in the Highly Symptomatic cluster. Our results have direct applicability to clinical practice in that patients can be taught to recognize concerning sympom profiles (e.g. seek prompt outpatient management before symptoms progress to occurring at rest).
The individuals with the highest rates of both ED visits and hospitalizations belong to the Highly Symptomatic cluster. Individuals in this cluster are likely to experience multiple symptoms, in particular shortness of breath with and without activity, exercise intolerance, palpitations, and fatigue at rest. These individuals are more likely to be obese and have persistent AF, heart failure, and coronary disease when compared to individuals in the Asymptomatic cluster. These results are congruent with prior research that identifies heart failure,33 coronary disease,7 and obesity35 as risk factors for AF symptoms. In contrast to our findings, prior research suggests that AF symptoms are more likely in individuals with paroxysmal (77% have symptoms) versus persistent AF (73%).36 An examination of specific symptoms by type of AF reveals that while some symptoms are more common in paroxysmal compared to persistent AF (palpitations, 79% versus 52% respectively), other symptoms are less likely (dyspnea, 23% versus 58% respectively).12 Heart failure has previously been shown to increase the risk of hospitalizations among patients with AF.10 Results of prior studies have been inconsistent in regards to differences in hospitalizations by type of AF, with some demonstrating that patients with persistent AF have more hospitalizations for AF than patients with paroxysmal AF36, 37 and others revealing similar hospitalization rates across the two classifications.10, 38 Our results augment current knowledge by providing a comprehensive and specific symptom and clinical profile of patients with an elevated rate of AF-related ED visits and hospitalizations.
Using the same data set (VAFR), two AF-specific symptom clusters (the At Rest and With Activity clusters) were previously identified using cluster analysis, an approach that clusters symptoms rather than individuals, resulting in mutually exclusive clusters of symptom variables.18 In the present latent class analysis, our approach was to cluster individuals, therefore the same symptom may be present in multiple clusters, but differ based on the probability of each Likert scale rating. The results of these two studies are complimentary, with both having a With Activity cluster, which is marked by the symptoms shortness of breath with activity and exercise intolerance. Further, the At Rest cluster from the cluster analysis is similar to the Highly Symptomatic cluster from the latent class analysis, with both including individuals who experience shortness of breath at rest, fatigue at rest, chest pain, and dizziness. The Highly Symptomatic cluster is additionally marked by shortness of breath with activity and exercise intolerance, which remains congruent with the cluster analysis findings, given that 51 out of the 56 individuals in the At Rest cluster also had the With Activity cluster.18 Neither the cluster analysis with VAFR18 nor the clusters identified in the present latent class analysis, align with the cluster analysis results conducted using SAFETY trial participants.19, 39 Reasons for the discrepancy likely include differences in sample recruitment (SAFETY recruited only from inpatient setting), inclusion criteria (SAFETY excluded patients with heart failure), and symptom measurement (SAFETY used a study specific tool whereas VAFR used the AFSS).19, 39
Gender Differences
The Highly Symptomatic cluster was equally split between males and females despite the fact that the overall study population was 67% male and 33% female. The With Activity cluster similarly had a greater proportion of females than the overall study population. These results are consistent with prior research which shows that women are more likely than men to experience a significant level of symptoms, negatively impacting their quality of life.40 VAFR participants did not complete quality of life measures, so we were not able to explore the association of cluster membership with quality of life outcomes. Future research examining the relationship between specific AF symptom profiles and quality of life would be beneficial in determining which patients have the greatest potential to benefit from symptom management interventions.
Age-based Differences
Age was not a factor that influenced membership in the Highly Symptomatic or With Activity clusters, although members of the Mild Diffuse cluster were approximately 2 years younger than Asymptomatic cluster members. Consistent with our findings, prior research on AF symptom clusters showed that age may vary for some, but not all, symptom clusters.19 In that study, AF patients with the cluster of chest pain and palpitations were younger than members of other clusters. These findings contradict the commonly held belief that AF symptoms are reduced with advancing age.
Implications for Practice
Rhythm control strateties (i.e. antiarrhythmic medications and ablation) are a primary means through which AF symptoms are managed. Therefore, it is not surprising that individuals in the Highly Symptomatic and With Activity clusters were more likely to be on a rhythm control strategy compared to individuals in the Asymptomatic cluster. In addition to rhythm control, individuals in the Highly Symptomatic or With Activity cluster many benefit from self-care interventions that have the potential to improve AF symptoms and monitoring, thereby reducing ED visits and hospitalizations. The goal of self-care is for patients to adequately monitor, maintain, and manage their health.41 For example, weight reduction, cardio-respiratory fitness, and cardiometabolic risk factor management reduce AF symptom burden, symptom severity, and arrhythmia recurrence, making weight reduction and physical activity meaningful self-care goals for individuals with symptomatic AF.42–44 The standard versus AF specific management strategy (SAFETY) trial compared standard care to nurse managed home and telephone-based follow up for hospitalized patients with AF, which included elements related to self-care such as patient/caregiver education and medication management, and resulted in an increased proportion of days alive and out of the hospital.39 Another important component of AF-specific self-care is heart rate monitoring, which can help patients identify the presence of AF when symptoms are vague or non-specific.6 Individuals with clinical profiles associated with increased rates of healthcare utilization (i.e. those with heart failure, obesity, and/or coronary disease who are highly symptomatic) are ideal candidates for AF-specific self-care interventions. However, to date there is a gap in the literature related to comprehensive self-care measures or interventions specific to these individuals.
Chest pain and palpitations were uncommon symptoms in this sample, despite prior reports indicating they are common symptoms of AF.12, 19 Chest pain was likely to be reported as absent or infrequent by members of every symptom cluster in our study. Palpitations, a symptom commonly associated with AF, had only a low to moderate probability in every cluster we identified. These findings are important since chest pain and palpitations are classic cardiac symptoms and patients may have difficulty interpreting the less cardiac-specific symptoms in this study (e.g. shortness of breath, fatigue). Lack of accurate symptom interpretation influences the response to symptoms, possibly delaying early intervention and prompt treatment.5, 6 Prompt recognition of symptoms and treatment in a non-urgent outpatient setting has the potential to reduce utilization of the ED and subsequent hospitalizations. Clinicians can apply this research to practice by ensuring that people with AF are educated and knowledgable about the non-specific symptoms that often occur with AF, and encouraged to seek early assistance with symptom management in order to avoid potentially avoidable ED visits and hospitalizations.6, 45 It is plausible that by seeking early outpatient treatment for symptoms, patients may be able to avoid progression to symptoms that occur at rest (shortness of breath at rest, fatigue at rest).
Limitations
Three limitations are worth noting. First, our AFSS healthcare utilization outcomes variables (AF-related ED visits and hospitalizations) were self-reported and were not verified with medical records. However, the AFSS was obtained by a trained study registered nurse either by telephone or in person. Consequently, patients with questions regarding their history of AF-related ED visits and hospitalizations had access to study nurses for clarification regarding accurate completion of the AFSS. Second, history of ablation was increased for all latent classes (in comparison to the asymptomatic class). Because of limitations related to our cross-sectional study design and our de-identified data set, we do not know the timing of ablations and what proportion of the hospitalizations reported on the AFSS may be for these (typically) planned admissions. Third, compared with other large scale AF registries,46, 47 our sample is younger, includes more males and Caucasians, and has lower rates of both heart failure and coronary artery disease. Some of these differences, such as heart failure and coronary disease, are likely related to the genetic focus of VAFR and the recruitment strategy of enrolling AF patients and their family members, which likely led to more individuals being enrolled in this registry who lack these particular AF risk factors.
Conclusion
We identified 4 AF-specific symptom clusters using latent class analysis and showed that membership in specific clusters is associated with increased ED visits and hospitalizations. Cluster membership is associated with several sociodemographic and clinical factors, most notably gender, AF type, heart failure, coronary disease, and BMI. The generalizability of these results remains unclear and an attempts to replicate the findings are warranted. Future research is warranted to verify whether symptom clusters can be used clinically to identify patients at an elevated risk for ED visits or hospitalizations, and whether interventions targeted towards these patients could reduce the rate of ED visits and unplanned hospitalizations, and improve quality of life.
Acknowledgments
Megan Streur was supported by the National Institute of Nursing Research (F31 NR015687 and T32 NR014833). M. Benjamin Shoemaker was supported in part by the National Institutes of Health grant K23 HL127704. The registry was supported by CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
We would like to acknowledge Dawood Darbar, MD and Dan Roden, MD for their leadership roles related to the Vanderbilt Atrial Fibrillation Registry.
Footnotes
No conflicts of interest to report.
Author Contributions
All authors meet the journal’s criteria for authorship and have approved the final article. Further, all those entitled to authorship are currently listed as authors. The authors contributions are as follows: Megan Streur wrote the first draft of the manuscript, was responsible for implementing feedback from each of the other authors, and was the primary individuals who designed the study, conducted the statistical analysis, and interpreted the analysis. Sarah Ratcliffe provided critical feedback on the manuscript, was involved in study design (especially in relation to statistical methods), and mentored the first author on the conduct and interpretation of statistical analysis. David Callans provided critical feedback on the manuscript and critical feedback in terms of the design of the study and interpretation of results. M. Benjamin Shoemaker provided critical feedback on the manuscript and was instrumental to the data acquisition. Barbara Riegel provided critical feedback on the manuscript, significant input regarding the overall study design, and critical feedback regarding the interpretation of results.
Contributor Information
Megan M. Streur, Email: sratclif@upenn.edu.
David J. Callans, Email: david.callans@uphs.upenn.edu.
M. Benjamin Shoemaker, Email: moore.b.shoemaker@Vanderbilt.edu.
Barbara J. Riegel, Email: briegel@nursing.upenn.edu.
References
- 1.van Wijk CM, Kolk AM. Sex differences in physical symptoms: the contribution of symptom perception theory. Soc Sci Med. 1997 doi: 10.1016/s0277-9536(96)00340-1. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong TS. Symptoms experience: a concept analysis. Oncol Nurs Forum. 2003 doi: 10.1188/03.ONF.601-606. [DOI] [PubMed] [Google Scholar]
- 3.Posey AD. Symptom perception: a concept exploration. Nurs Forum. 2006 doi: 10.1111/j.1744-6198.2006.00047.x. [DOI] [PubMed] [Google Scholar]
- 4.Teel CS, Meek P, McNamara AM, et al. Perspectives unifying symptom interpretation. Image J Nurs Sch. 1997 doi: 10.1111/j.1547-5069.1997.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 5.McCabe PJ, Rhudy LM, DeVon HA. Patients’ experiences from symptom onset to initial treatment for atrial fibrillation. J Clin Nurs. 2015 doi: 10.1111/jocn.12708. [DOI] [PubMed] [Google Scholar]
- 6.McCabe PJ, Rhudy LM, Chamberlain AM, et al. Fatigue, dyspnea, and intermittent symptoms are associated with treatment-seeking delay for symptoms of atrial fibrillation before diagnosis. Eur J Cardiovasc Nurs. 2016 doi: 10.1177/1474515115603901. [DOI] [PubMed] [Google Scholar]
- 7.Rienstra M, Lubitz SA, Mahida S, et al. Symptoms and functional status of patients with atrial fibrillation: state of the art and future research opportunities. Circulation. 2012 doi: 10.1161/CIRCULATIONAHA.111.069450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald AJ, Pelletier AJ, Ellinor PT, et al. Increasing US emergency department visit rates and subsequent hospital admissions for atrial fibrillation from 1993 to 2004. Ann Emerg Med. 2008 doi: 10.1016/j.annemergmed.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Ball J, Carrington MJ, McMurray JJV, et al. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. Int J Cardiol. 2013 doi: 10.1016/j.ijcard.2012.12.093. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg BA, Kim S, Fonarow GC, et al. Drivers of hospitalization for patients with atrial fibrillation: Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Am Heart J. 2014 doi: 10.1016/j.ahj.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman JV, Simon DN, Go AS, et al. Association Between Atrial Fibrillation Symptoms, Quality of Life, and Patient Outcomes: Results From the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Circ Cardiovasc Qual Outcomes. 2015 doi: 10.1161/CIRCOUTCOMES.114.001303. [DOI] [PubMed] [Google Scholar]
- 12.Lévy S, Maarek M, Coumel P, et al. Characterization of different subsets of atrial fibrillation in general practice in France: the ALFA study. The College of French Cardiologists. Circulation. 1999 doi: 10.1161/01.cir.99.23.3028. [DOI] [PubMed] [Google Scholar]
- 13.Kim H, McGuire DB, Tulman L, et al. Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs. 2005 doi: 10.1097/00002820-200507000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Miaskowski C, Dodd M, Lee K. Symptom clusters: the new frontier in symptom management research. J Natl Cancer Inst Monographs. 2004 doi: 10.1093/jncimonographs/lgh023. [DOI] [PubMed] [Google Scholar]
- 15.Barsevick AM, Whitmer K, Nail LM, et al. Symptom cluster research: conceptual, design, measurement, and analysis issues. J Pain Symptom Manage. 2006 doi: 10.1016/j.jpainsymman.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Barsevick AM. The elusive concept of the symptom cluster. Oncol Nurs Forum. 2007 doi: 10.1188/07.ONF.971-980. [DOI] [PubMed] [Google Scholar]
- 17.Kim H, Abraham IL. Statistical approaches to modeling symptom clusters in cancer patients. Cancer Nurs. 2008 doi: 10.1097/01.NCC.0000305757.58615.c8. [DOI] [PubMed] [Google Scholar]
- 18.Streur M, Ratcliffe S, Shoemaker MB, et al. Atrial Fibrillation Symptom Clusters Increase Hospitalizations and Emergency Department Visits. American Heart Association. 2016 [Google Scholar]
- 19.Streur M, Ratcliffe SJ, Ball J, et al. Symptom Clusters in Adults With Chronic Atrial Fibrillation. J Cardiovasc Nurs. 2017 doi: 10.1097/JCN.0000000000000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darbar D, Motsinger AA, Ritchie MD, et al. Polymorphism modulates symptomatic response to antiarrhythmic drug therapy in patients with lone atrial fibrillation. Heart Rhythm. 2007 doi: 10.1016/j.hrthm.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abraham RL, Yang T, Blair M, et al. Augmented potassium current is a shared phenotype for two genetic defects associated with familial atrial fibrillation. J Mol Cell Cardiol. 2010 doi: 10.1016/j.yjmcc.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darbar D, Kannankeril PJ, Donahue BS, et al. Cardiac sodium channel (SCN5A) variants associated with atrial fibrillation. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.107.757955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorian P, Jung W, Newman D, et al. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol. 2000 doi: 10.1016/s0735-1097(00)00886-x. [DOI] [PubMed] [Google Scholar]
- 25.Dorian P, Burk C, Mullin CM, et al. Interpreting changes in quality of life in atrial fibrillation: how much change is meaningful? Am Heart J. 2013 doi: 10.1016/j.ahj.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 26.Dorian P, Guerra PG, Kerr CR, et al. Validation of a new simple scale to measure symptoms in atrial fibrillation: the Canadian Cardiovascular Society Severity in Atrial Fibrillation scale. Circ Arrhythm Electrophysiol. 2009 doi: 10.1161/CIRCEP.108.812347. [DOI] [PubMed] [Google Scholar]
- 27.Dorian P, Paquette M, Newman D, et al. Quality of life improves with treatment in the Canadian Trial of Atrial Fibrillation. Am Heart J. 2002 doi: 10.1067/mhj.2002.122518. [DOI] [PubMed] [Google Scholar]
- 28.Linzer DA, Lewis J. poLCA: an R package for polytomous variable latent class analysis. Journal of Statistical Software (Internet) 2011 [Google Scholar]
- 29.Linzer DA, Lewis J. poLCA: Polytomous Variable Latent Class Analysis. [accessed December 12, 2016];R package version 1.4. 2013 http://dlinzer.github.com/poLCA.
- 30.R Development Core Team. [accessed Accessed December 12, 2016];R: A language and environment for statistical computing. 2008 http://www.R-project.org.
- 31.January CT, Wann LS, Alpert JS, et al. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014 doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 32.Paquette M, Roy D, Talajic M, et al. Role of gender and personality on quality-of-life impairment in intermittent atrial fibrillation. Am J Cardiol. 2000 doi: 10.1016/s0002-9149(00)01077-8. [DOI] [PubMed] [Google Scholar]
- 33.Silva-Cardoso J, Zharinov OJ, Ponikowski P, et al. Heart failure in patients with atrial fibrillation is associated with a high symptom and hospitalization burden: the RealiseAF survey. Clin Cardiol. 2013 doi: 10.1002/clc.22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dziak JJ, Coffman DL, Lanza ST, et al. Sensitivity and specificity of information criteria. Jun 27; doi: 10.1093/bib/bbz016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garimella RS, Sears SF, Gehi AK. Depression and Physical Inactivity as Confounding the Effect of Obesity on Atrial Fibrillation. Am J Cardiol. 2016 doi: 10.1016/j.amjcard.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Nieuwlaat R, Capucci A, Camm AJ, et al. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2005 doi: 10.1093/eurheartj/ehi505. [DOI] [PubMed] [Google Scholar]
- 37.Le Heuzey J, Paziaud O, Piot O, et al. Cost of care distribution in atrial fibrillation patients: the COCAF study. Am Heart J. 2004 doi: 10.1016/s0002-8703(03)00524-6. [DOI] [PubMed] [Google Scholar]
- 38.DeVore AD, Hellkamp AS, Becker RC, et al. Hospitalizations in patients with atrial fibrillation: an analysis from ROCKET AF. Europace. 2016 doi: 10.1093/europace/euv404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart S, Ball J, Horowitz JD, et al. Standard versus atrial fibrillation-specific management strategy (SAFETY) to reduce recurrent admission and prolong survival: pragmatic, multicentre, randomised controlled trial. Lancet. 2015 doi: 10.1016/S0140-6736(14)61992-9. [DOI] [PubMed] [Google Scholar]
- 40.Piccini JP, Simon DN, Steinberg BA, et al. Differences in Clinical and Functional Outcomes of Atrial Fibrillation in Women and Men: Two-Year Results From the ORBIT-AF Registry. JAMA Cardiol. 2016 doi: 10.1001/jamacardio.2016.0529. [DOI] [PubMed] [Google Scholar]
- 41.Riegel B, Jaarsma T, Strömberg A. A middle-range theory of self-care of chronic illness. ANS Adv Nurs Sci. 2012 doi: 10.1097/ANS.0b013e318261b1ba. [DOI] [PubMed] [Google Scholar]
- 42.Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013 doi: 10.1001/jama.2013.280521. [DOI] [PubMed] [Google Scholar]
- 43.Pathak RK, Elliott A, Middeldorp ME, et al. Impact of CARDIOrespiratory FITness on Arrhythmia Recurrence in Obese Individuals With Atrial Fibrillation: The CARDIO-FIT Study. J Am Coll Cardiol. 2015 doi: 10.1016/j.jacc.2015.06.488. [DOI] [PubMed] [Google Scholar]
- 44.Pathak RK, Middeldorp ME, Meredith M, et al. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY) J Am Coll Cardiol. 2015 doi: 10.1016/j.jacc.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 45.McCabe PJ, Douglas KV, Barton DL, et al. Feasibility Testing of the Alert for AFib Intervention. West J Nurs Res. 2016 doi: 10.1177/0193945916656609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinberg BA, Holmes DN, Ezekowitz MD, et al. Rate versus rhythm control for management of atrial fibrillation in clinical practice: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Am Heart J. 2013 doi: 10.1016/j.ahj.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steinberg BA, Shrader P, Thomas L, et al. Off-Label Dosing of Non-Vitamin K Antagonist Oral Anticoagulants and Adverse Outcomes: The ORBIT-AF II Registry. J Am Coll Cardiol. 2016 doi: 10.1016/j.jacc.2016.09.966. [DOI] [PubMed] [Google Scholar]


