Abstract
The take-home pathway is a significant source of organophosphate pesticide exposure for young children (3–5 years old) living with an adult farmworker. This avoidable exposure pathway is an important target for intervention. We selected 24 agricultural communities in the Yakima Valley of Washington State and randomly assigned them to receive an educational intervention (n = 12) to reduce children’s pesticide exposure or usual care (n = 12). We assessed exposure to pesticides in nearly 200 adults and children during the pre and post-intervention periods by measuring metabolites in urine. We compared post and post-intervention exposures by expressing the child’s pesticide metabolite concentration as a fraction of the adult’s concentration living in the same household, because the amount of pesticides applied during the collection periods varied. Exposures in our community were consistently higher, sometimes above the 95th percentile of the exposures reported by the National Health and Nutrition Examination Survey (NHANES). While intervention and control communities demonstrated a reduction in the ratio of child to adult exposure, this reduction was more pronounced in intervention communities (2.7 fold, p<0.001 compared to 1.7 fold, p=0.052 for intervention and control, respectively). By examining the child/adult biomarker ratio, we demonstrated that our community-based intervention was effective in reducing pesticide exposure to children in agricultural communities.
1. Introduction
Agriculture is a key component of the economy in Washington State. During this intervention study (1999–2002), organophosphate pesticides (OP) were widely applied to pome fruit (e.g. apples and pears). In 2002, agricultural production in Washington State totaled $5.56 billion, with apple crops accounting for $1.02 billion of that sum or 19% of total agricultural value produced [1]. Washington produced more apples, pears, and cherries than any other US state in 2002. Collectively, production of three fruit crops – apples, cherries, and pears – totaled approximately $1.29 billion in 2002 [1]. More than 400,000 pounds of three different dimethyl OP insecticides (chlorpyrifos, azinphos methyl, phosmet) were applied between 1999 and 2003 to Washington’s pome fruit crops. Evidence suggests that OPs are harmful to the health of these communities, particularly children’s neurodevelopment [2–4].
OP residue from crops and the ambient environment have been shown to travel home with the farmworkers, where they are introduced into the household environment [5–7]. The occupational take-home pathway can lead to pesticide exposure in children through direct contact with parents’ contaminated clothes, skin or hair, contamination of children’s clothing through shared laundering, and ingestion, inhalation or having dermal contact with house dust contaminated with pesticides brought home by farmworkers. A recent review of non-occupational pesticide exposure pathways for women living in agricultural communities found that the spousal occupation was significantly correlated to pesticide exposure in more studies than agricultural drift or dietary exposure pathways [8], however, the authors lamented the lack of seasonal exposure variability available in these studies. Our Community-Based Participatory Research Project (CBPR) has characterized the seasonal variability in dust and urinary biomarkers for pesticide metabolites [7,9]. We have also characterized dietary exposure to pesticides in this community, and while we were able to detect differences in exposure due to diet, household occupational status (if there is at least one farmworker in the household) was still the largest contributor to pesticide exposure [10]. These studies have pinpointed the seasons during which the occupational take-home pathway for pesticide exposure may be highest in families living in Yakima Valley, Washington. The occupational take-home pathway provides a unique opportunity for families to take action to reduce pesticide exposures for children and other non-farmworker family members. Figure 1 outlines the occupational take-home pathway assessed in our CBPR project. By interrupting the exposure pathways, shown by the solid arrows, we hypothesized that farmworkers’ children would experience lower pesticide exposure. Breaking or reducing the take-home pathway represents a feasible and effective way to protect agricultural families from further OP exposure.
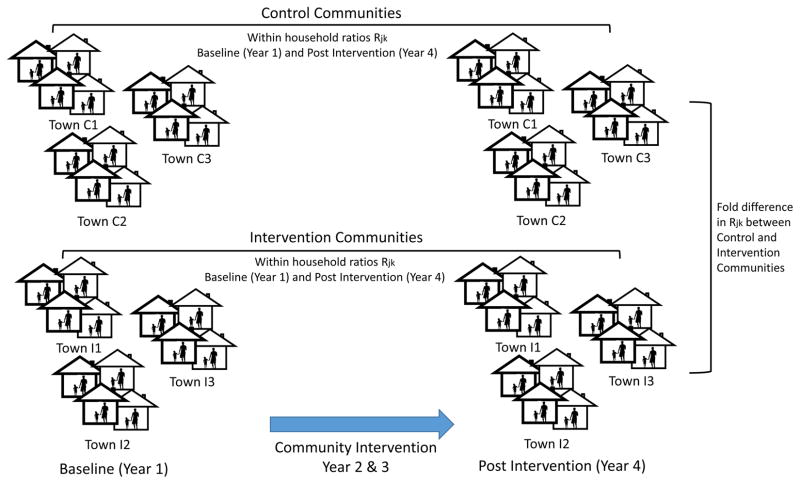
Figure 1.
Conceptual model for assessing children’s pesticide exposure via the take-home pathway. In this model, within household ratios (Rjk) are compared between baseline (Year 1) and post intervention (Year 4) within each group. Fold difference in Rjk between Control and Intervention communities are compared. Each group included 12 towns.
Using our CBPR program, we have explored the potential for reducing childhood OP exposure by intervening in the take-home exposure pathway [11]. Previously, we have observed increases in exposure prevention behaviors, related to breaking the take-home pathway following the community level education intervention [12]. However, we did not observe a reduction in the concentrations of OPs and metabolites in dust and urine, respectively [13]. These conflicting results led us to re-evaluate whether a reduction in exposure from improved exposure prevention behaviors may be masked by other variables, such as fluctuations in pesticide use over time. This paper evaluates the intervention’s efficacy in interrupting the occupational take-home pathway by controlling each child’s exposure by the exposure of the adult in the household measured through the ratio of the child to adult DMTP in urine. We chose this method of analysis because the exposures of adults can vary considerably between years and within an agricultural season. Pesticide use is adjusted in each orchard on a week-by-week basis depending on when insects harmful to a particular crop are hatching. The amounts of OPs used each year has been shown to effect the amount of OP metabolites observed in the urine of children in the Yakima Valley [14]. Thus, an overall change in pesticide exposure in children may not be indicative of a change in the take-home pathway. The child to adult ratio also provides a direct measure of the effectiveness of the community intervention since a lower ratio shows that the child has a relatively lower exposure to the OPs being brought home by the adult from work in pome fruit orchards. The child to adult ratio also adjusts for the particular work schedule and location of the adult, which can be highly variable during an agricultural season.
2. Methods
Setting
This intervention study occurred in the Yakima Valley of Washington State, a major agricultural region of the Western United States, where apples and pears (pome fruits) as well as peaches, cherries, grapes, and hops (non-pome fruits) are the primary crops. Organophosphate pesticides (OP) are in annual use on those crops, making this agricultural area appropriate for pesticide exposure research. Yakima County is home to 45.8% of Washington State’s Hispanic population. Similarly, Yakima Valley has a high proportion of Hispanics within the county population, many of whom are agricultural workers [11,15,16]. Approximately 90% of our study participants are of Hispanic descent [12].
Study Population
A full description of the study population can be found in [11,12]. Briefly, a total of 24 community groups were recruited for this study, including 16 communities and 8 labor camps. These 24 participating communities were randomized into intervention and control subgroups, stratified by community size. Within each community, farmworkers were chosen based upon having a child 2–6 years of age resident in their home. Eligible farmworkers were consented and interviewed, and urine samples were collected from the farmworker and referent child, and dust samples were collected from the farmworker home and commuter vehicle. Following baseline data collection, a two-year multi-faceted educational intervention that focused on reducing child pesticides exposures was delivered. In year 4, a post-intervention cross-sectional survey of farmworkers was administered and urine and dust samples were collected in each of the 24 participating communities. For the post-intervention survey in most cases we surveyed new households so that children were in the same age range as our baseline survey [11,12]. The study protocol and procedures were approved by the Human Subjects Review Board at the University of Washington (No. 98-6567-C) and the Institutional Review Board at the Fred Hutchinson Cancer Research Center (No. 5101).
Community Intervention Program
A community intervention was developed using a community based participatory research approach with input from a Community Advisory Board comprised of local residents. More details about this approach can be found in [11]. Intervention was a multifaceted community education campaign that included health fairs, festivals, and block parties. Local media also assisted in dissemination of intervention materials, which were designed to inform community members of the risks of childhood exposure to pesticides, the symptoms of pesticide intoxication, and how to protect oneself and family from pesticides. For instance, laundering adult work clothes separately from children’s clothing was a common preventive step included in the intervention. Community centers like schools, churches, adult citizenship and English language courses, workplaces, community clinics, and the farmworker union served as platforms for the intervention program. We promoted group discussions in these environments, and emphasized the importance of protecting children by preventing pesticides from being tracked into the home after work. To empower participants for immediate action, we distributed sample packets of detergent for contaminated work clothes, plastic bins for storing work boots, shower kits, and other deliverables to participants after community meetings. Our teams also recommended regular vacuuming of the car and home as well as general household cleaning [13].
Bilingual health educators (promotoras) organized in “home health parties” throughout the intervention communities to facilitate preventive pesticide exposure dialog, and more than 1,100 of these parties were held during the 2-year intervention period. Other volunteer promotoras remained visible throughout the community by visiting grocery stores and going door-to-door to speak with farmworkers and their families. Promotoras distributed the laundry kits, shower kits, and the other tools distributed at larger community intervention events.
Sample Collection & Study Design
This study is based on the broader experimental plan and biomonitoring sample design. Workplace exposures observed in adults can be tracked home to children and accumulate in house and vehicle dust. We have measured OP concentrations in house and vehicle dust and urinary metabolites of OPs in these communities [7,13]. Figure 1 illustrates our study design and analytical framework.
We chose to focus this investigation on pome fruit worker exposure during the thinning season because our previous assessments indicated this group and season have the highest exposures [17]. Further, our Community Advisory Board emphasized the need for exposure prevention resources among pome fruit workers as they were not covered by the same protective practices as pesticide applicators or handlers. We invited adults with children between the ages 2–6 in the same household to participate in the study. We visited these households to collect environmental dust samples and urine samples from both adults and children before and after the intervention period. Sampling protocols are detailed in an earlier publication [18]. Briefly, we collected three urine samples from one adult and one child of each eligible household before and after the intervention period.
The urine collection took place between June 1999 and October 1999 for baseline and between June 2002 and September 2002 for post-intervention surveys. Collections in each season were separated by a minimum of 3 days and at maximum of 2 weeks from one child and one farmworker. Both timeframe coincided with the thinning (spring and early summer) and harvest (fall) seasons for pome fruits when farmworkers had direct contact with crops. During the thinning season farmworkers remove buds or small fruit from trees so that bigger fruit can grow. During this time, farmworkers had direct contract with trees that may have been recently treated with pesticides. In addition, the thinning season was when dimethyl OP pesticides, such as malathion, azinphos methyl, and phosmet were sprayed to control pests. Later studies in this community have observed that the thinning and harvest seasons are the highest exposure periods for farmworkers [7,9,19].
Samples were refrigerated immediately after being produced and transferred to the field laboratory in coolers. At the laboratory, samples were refrigerated until all samples from a participant were provided. At that point, equal volumes of the independent urine samples (approximately 15 mL each) were pooled for each individual. Small tubes of pooled urine were drawn, frozen and shipped on ice to the laboratory at the University of Washington and stored at −10°C until analysis. Urine samples were analyzed for three OP metabolites – dimethylphosphate (DMP), dimethylthiophosphate (DMTP), and dimethyldithiophosphate (DMDTP). These are metabolites of azinphosmethyl, phosmet and malathion, which were the most commonly applied pesticides during our collection period in the Yakima Valley.
Statistical Analysis
To evaluate the take-home pathway, we modeled the metabolite data from each household with a multivariate log-normal distribution using Bayesian Markov chain Monte Carlo (MCMC) method known as Gibbs sampling. This distribution model was used to study the associations between the adult and child metabolite concentrations in the conceptual model shown in Figure 1. This method allowed us to use all the observations of metabolite concentrations in the dataset, even the observations below the level of detection (LOD), treating them as being left censored[14]. Based upon quantile-quantile plots we found that the log of the data values above the limit of detection was approximately normally distributed. We used WinBUGS software [20] for MCMC estimation of the parameters of a multivariate log-normal distribution of the metabolites. We calculated the ratio of the child to adult DMTP urine concentrations for each household from these simulations to find the distribution of the ratio values for the pre-intervention and post-intervention samples. We estimated geometric means of each metabolite, population geometric standard deviations, correlations between OPs and standard errors of the parameters. The model is described by:
| (1) |
where MVN6 is a six-dimensional multivariate normal distribution of the metabolites DMP, DMTP, DMDTP for adults and children. Xjk is the vector of the k=1,…,6 measured metabolite concentrations of the jth household, with k=1,2,3 (DMP, DMTP, DMDTP) for adults and k=4,5,6 (DMP, DMTP, DMDTP) for children. θk is the vector of the estimated mean concentrations in urine of the metabolites, and Σ is the estimated variance–covariance matrix (6×6) among the adult and child metabolites. The number of households is nj. Population standard deviations for each metabolite and correlations between metabolites were estimated by the variance-covariance matrix Σ. Separate multivariate Normal distributions, MVN6, were estimated for households in pre and post intervention years. We discarded the first 5,000 simulations to initialize the Markov Chain and then retained 100,000 simulations to describe the joint distribution among the dimethyl metabolites. The child to adult ratios were calculated for each simulation and household by Rjk=X j,k+3 / X j,k for j=1,…,nj, and k= 1,..,3 (DMP, DMTP, DMDTP). When a value was below the limit of detection then its simulated value from the MCMC simulation was used.
Based upon plots of the simulations, the Markov Chain rapidly mixed and was stable [21]. Censored values are treated in each simulation as being from the lower tail of a multivariate log-normal distribution with an upper cutoff at the limit of detection and the shape of the distribution determined by the parameter values for the simulation. This model was used to estimate p-values for the differences between child to adult ratios. The multivariate log-normal distributions estimated separate values for adult and child, the value for the ratio was calculated by dividing the child value by the adult value for each simulation.
We focused our statistical analysis on DMTP because it results from exposure to dimethyl OPs (azinphosmethyl, phosmet and malathion), which were targeted by our sample collection timeframe and thus had higher concentrations in our samples [13,22]. DMTP was above the LOD in 87.5% and 85.5% of child urine samples in the pre and post intervention periods, respectively. Adult urine samples had higher rates of detection (94% and 90% in the pre and post intervention years, respectively). The ratios from the simulations were then used to estimate the means, standard errors, correlations and p-values for the ratios.
3. Results
Using our conceptual model (Figure 1), we were able to design and implement sample collection in collaboration with our Community Advisory Board from 197 adults and 186 children in year 1 and 187 adults and 172 children in year 4. We successfully collected and analyzed 383 urine samples in pre and 359 in post intervention period.
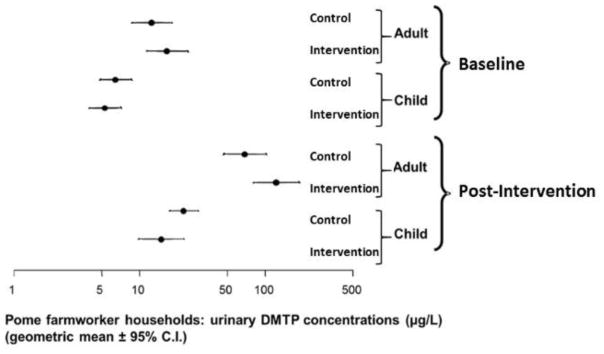
Mean values for adult urinary DMTP in pre intervention period were, on average 13.3 μg/L for the control group and 17.6 μg/L for intervention the group. Both values are levels between 1999–2000 National Health and Nutrition Examination Survey (NHANES) 75th percentile (11.0 μg/L) and 90th percentile (38.0 μg/L) (NHANES Feb. 2015) for the population as a whole. Child urinary DMTP concentrations in the pre intervention period were 6.8 μg/L for the control and 5.7 μg/L for intervention the group. While children in this study were younger than NHANES’ youngest age group of 6 and 11 years old, our children’s values were higher than NHANES’ geometric mean of 2.72 μg/L for 1999–2000. For the post intervention period, adult urinary DMTP concentrations were on average 73.1 μg/L for the control and 130.8 μg/L for the intervention group. Both of these values are above 95th percentile (48.0 μg/L) of 2001–2002 NHANES’. Child urinary DMTP in the post intervention period were 23.8 μg/L for the control and 15.9 for the intervention group. Our children’s values in the post intervention period remained at the level between NHANES’ 75th and 95th percentiles for 2001–2002 (8.33 μg/L and 28.4 μg/L). Part of the DMTP may be due to preformed metabolites [23]. However, the concentrations seen in this study of DMTP concentrations are much higher than in NHANES. Additional longitudinal studies that we have conducted with this population have also shown that DMTP concentrations decline during the off season when farmworkers are not in the orchards suggesting that higher exposures during the thinning season are from OPs being used in the orchards [14]. The level of DMTP in the non-farmworker population does not show these seasonal shifts.
The concentration of DMTP in urine was higher in the post intervention year relative to the pre intervention year for both control and intervention communities and for both adults and children (Figure 2).
Figure 2.
Concentrations of DMTP in the urine of adults and children for control and intervention communities in pre and post intervention years. Values and confidence intervals were calculated using the model in (1). The concentrations of DMTP in urine were higher in the post intervention year than the pre intervention year for both control and intervention communities and for both adults and children.
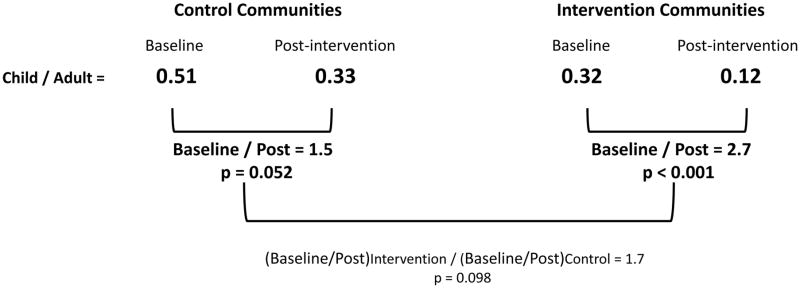
The ratio of the child urine concentration to the adult urine concentration in the post intervention year and the pre intervention year (Figure 3) shows that for all of the groups the child to adult ratio is less than 1, indicating that the child’s exposure is significantly less than the adult exposure (p<0.001). We also observed a decrease in the child/adult ratio for both the control and intervention groups. When we compared the decrease in the child/adult ratio for the control group we found a 1.7-fold decrease from the baseline value of 0.51 to the post intervention year value of 0.33 (p=0.052). When the same comparison is made for the intervention group we observed a 2.7-fold decrease from the baseline value of 0.32 to the post-intervention value of 0.12 (p<0.001). This indicates that the intervention significantly reduced children’s exposure to OPs, relative to adults through the take home pathway. The intervention communities were 1.7 times the control communities in reduction which is significant at the p=0.098 level.
Figure 3.
Take-Home Pathway Intervention Results based on the ratio of the child to adult urine concentration of DMTP. The p-values for “Post/Baseline” and “Intervention/Control” are for testing whether these values are different from a value of 1.0 which would indicate no effect. The results of using the ratio of the child urine concentration to the adult urine concentration to compare the post intervention year to pre intervention year shows significant decrease in the ratio in both the control and intervention communities following the intervention, but more pronounced in intervention communities.
4. Discussion
We assessed the impact of a community-based intervention to reduce childhood exposure to pesticides by blocking the occupational take-home pathway. By using the ratio of common pesticide metabolites in the child to parent urinary samples, we were able to assess the efficacy of the intervention relative to changes in pesticide use at baseline and post-intervention. These analyses are consistent with our original hypothesis, that workers in the intervention groups would bring less pesticide exposures home to their families than workers in the control groups [5,22]. The ratio of child to adult urinary DMTP concentrations was a useful way to assess the OP take-home exposure pathway.
Separating the variability in pesticide use and work patterns between pre and post intervention years can be complex, as we observed in this study. All the urinary pesticide metabolite concentrations shifted upward in the post-intervention year. The shift for the control community adults provides us with a measure of how much greater the exposure was in the sampling period for the post-intervention compared to the baseline year, an increase of 5.1 fold. The amount of pesticide use varies both within and across seasons and depends upon the emergence of insects which changes from year to year; therefore we could only match our sampling times to the general period of pesticide use rather than to spray events. [11,13,14,24]. Increases in pesticide use has the potential to mask exposure intervention effectiveness. The overall increase in urinary DMTP we observed between pre and post intervention years was not sufficiently granular to be able to interpret the take-home exposure pathway or our intervention alone [11].
Urinary DMTP concentrations were higher in the post intervention year in both control and intervention communities. The higher DMTP concentrations in urine may be reflective of the timing of sample collection [11]. For example, we may have collected post intervention samples at a time when larger amounts of dimethyl OPs were being applied, thus indicating differences in seasonal use of dimethyl OPs [11]). According to a newsletter published by Washington State University, the average date of last spray of insecticides in apple orchards between 1989 and 2000 was mid-July (July 10th) with last date ranging from June 27 and August 13. Our pre intervention samples were collected in June-October 1999 and post intervention samples were collected in June-September, 2002, thus potentially explaining some of the variability surrounding urinary DMTP concentrations[5,22]. Unfortunately, pesticide use statistics do not contain seasonal breakdowns in Washington State. Thus, it is difficult to know how pesticide use varied during the periods sampled in this study.
In this analysis, we examined the effects of a community based intervention [12,13] specifically on interrupting the take-home pathway. Other educational interventions aimed at reducing pesticide exposure in farmworkers have focused on occupational pathways [25–28]. Many times the effectiveness of the intervention is measured by knowledge retention and behavioral changes. Few studies have assessed the impact of educational interventions using biomarkers for pesticide exposure. Bradman et al. (2009) measured biomarkers in urine as well as the parent compound on clothing. While the difference in pesticide metabolites between the control and treatment groups was insignificant (0.01 fold reduction in malathion dicarboxylic acid in urine between control and treatment groups), they were able to observe reductions associated with protective behaviors, such as glove wearing [29]. Reducing parental exposure has implications for the take-home pathway, but without assessing change in children’s exposure, it is difficult to know whether this intervention would successfully reduce children’s exposure. Further, the insignificant change in pesticide metabolites in urine between the control and intervention group observed by Bradman et al. 2009 may have been associated with a significant reduction in the take-home pathway that we identify in this paper.
Salvatore et al. (2015) measured children’s urinary metabolite concentrations and pesticide residues in the home among participants that did or did not receive a home based health worker intervention. Findings showed increases in concentrations of total dimethyl metabolites in urine among control group children while intervention group children showed a reduction following the intervention period (0.92 fold reduction in the intervention group and 1.5 fold increase in the control group) [30]. The increase observed in the control group shows how drastically pesticide exposure can change over time. Our method of controlling for changes in pesticide use over time by using the parental urine samples shows much greater sensitivity, as we report a 2.7 fold reduction in the ratio of child to adult exposure. By using the ratio of child to adult urinary pesticide concentrations, it is possible to control for overall changes in pesticide use and better assess the effectiveness of interventions targeting the take-home pathway.
The 2.7 fold reduction in the ratio of child to adult pesticide exposure observed in our study represent a significant reduction in children’s pesticide exposure. Previous analysis of this intervention study did not incorporate the variability in exposures between years and found no significant differences in urinary DMTP concentrations in adults and children following the educational intervention [13]. Our current analysis controls for the differences between years by using the ratio of child to adult urinary DMTP concentrations. This approach specifically targets the occupational take-home pathway because it analyzes the fraction of adults’ exposure that reaches the child. When compared to the fold changes identified in Salvatore (2015) and Bradman (2009), is more sensitive to changes in the take-home pathway as it can control for changes in parental exposure.
This community-based intervention was designed to provide a simple and effective message for interrupting the take-home pathway. It is possible that the intervention materials were distributed to participants in control communities by word of mouth. This occurrence could explain why we observed a reduction (though not statistically significant) in the child/adult metabolite ratios from year 1 to year 4 in the control communities. Strong et al. (2009) observed behavioral changes in both control and intervention communities following this intervention. For example, both groups were more likely to change out of work clothes promptly when returning home, however, the intervention group was also more likely to remove work shoes when entering the home. The increase in behaviors to reduce the take home pathway in both communities, may explain the reduction in control community exposures. Despite this, intervention communities still demonstrated a greater improvement in behaviors related to reducing the take-home pathway and a more significant reduction in the fraction of parental exposure brought home to children. Our analysis relied on data from cross-sectional samples of workers and children at the pre-and post-intervention time points, raising the possibility that workers who received the intervention were not included in the post-intervention assessment and other workers who may not have received the intervention were. This would lead to an attenuation of our estimates of effectiveness.
Strengths of this study design include the biomarker assessment of both adults and children during the pre and post intervention time periods. Additionally, we collected samples multiple times within each season, allowing for the characterization of variability in exposure. This is particularly important when assessing exposure to environmental chemicals with short half-lives. As the field of environmental health moves forward, more complex models are needed to integrate exposures across multiple lifestages, assessment methods and cohorts [31,32]. The recent NIH effort to integrate data across cohorts as part of the Environmental Influences on Child Health Outcomes (ECHO) program will require exposure assessment standardization techniques, such as the methods presented in this study. By using the ratio of child to adult exposure, other environmental changes that occurred during the study period are controlled.
5. Conclusion
Our study evaluated the impact of a community intervention program by quantifying child DMTP levels as a function of adult DMTP levels from the same household. Both Intervention and Control communities demonstrated a reduction in the ratio of child to adult exposure although this reduction was more pronounced in intervention communities (2.7-fold, p<0.001; 1.5, p=0.052 for intervention and control, respectively). The comparison of these two reductions shows that the reduction in the intervention communities are 1.7-fold greater than the control community was significant at p=0.098. Using the child to adult ratio allows us to better characterize and assess exposure of children since it provides a measure that provides much better control over annual and within season fluctuations in pesticide use. When comparisons are made using annual averages such as in Figure 2 the fluctuations in pesticide use during the sampling periods in each year prevent our ability to see important changes in how children’s exposures are different by year or season.
We estimate a probability of p < 0.001 that our community intervention program was responsible for that reduction in child DMTP levels relative to adult DMTP levels. The intervention program successfully disrupted the OP take-home pathway. Future studies should address reducing childhood OP exposure by other pathways while simultaneously accounting for the take-home pathway. Our intervention program is a promising addition to pesticide exposure prevention strategies in agricultural communities.
Supplementary Material
Acknowledgments
[This publication was made possible by grants PO1 ES009601 from NIEHS, and EPA RD-83170901, RD826886, RD83451401, RD-83273301.]
Footnotes
The authors have no competing financial interests to declare.
References
- 1.WSDA. Agriculture: A Cornerstone of Washington’s Economy. 2014 Available from: http://agr.wa.gov/AgInWA/
- 2.Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, et al. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ Health Perspect. 2011;119(8):1189–95. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, et al. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect. 2011;119(8):1182–8. doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, et al. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ Health Perspect. 2011;119(8):1196–201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coronado GD, Vigoren EM, Thompson B, Griffith WC, Faustman EM. Organophosphate pesticide exposure and work in pome fruit: evidence for the take-home pesticide pathway. Environ Health Perspect. 2006;114(7):999–1006. doi: 10.1289/ehp.8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson B, Coronado GD, Grossman JE, Puschel K, Solomon CC, Islas I, et al. Pesticide take-home pathway among children of agricultural workers: study design, methods, and baseline findings. J Occup Environ Med. 2003;45(1):42–53. doi: 10.1097/00043764-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Thompson B, Griffith WC, Barr DB, Coronado GD, Vigoren EM, Faustman EM. Variability in the take-home pathway: farmworkers and non-farmworkers and their children. Journal of exposure science & environmental epidemiology. 2014;24(5):522–31. doi: 10.1038/jes.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deziel NC, Friesen MC, Hoppin JA, Hines CJ, Thomas K, Freeman LE. A review of nonoccupational pathways for pesticide exposure in women living in agricultural areas. Environ Health Perspect. 2015;123(6):515–24. doi: 10.1289/ehp.1408273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith MN, Workman T, McDonald KM, Vredevoogd MA, Vigoren EM, Griffith WC, et al. Seasonal and occupational trends of five organophosphate pesticides in house dust. Journal of exposure science & environmental epidemiology. 2016 doi: 10.1038/jes.2016.45. [DOI] [PubMed] [Google Scholar]
- 10.Holme F, Thompson B, Holte S, Vigoren EM, Espinoza N, Ulrich A, et al. The role of diet in children’s exposure to organophosphate pesticides. Environ Res. 2016;147:133–40. doi: 10.1016/j.envres.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson B, Coronado GD, Grossman JE, MDKP, Solomon IIslas CC, et al. Pesticide Take-Home Pathway among Children of Agricultural Workers: Study Design, Methods, and Baseline Findings. Journal of Occupational and Environmental Medicine. 2003;45(1):42–53. doi: 10.1097/00043764-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Strong LL, Thompson B, Koepsell TD, Meischke H, Coronado GD. Reducing the take-home pathway of pesticide exposure: behavioral outcomes from the Para Ninos Saludables study. J Occup Environ Med. 2009;51(8):922–33. doi: 10.1097/JOM.0b013e3181ad4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson B, Coronado GD, Vigoren EM, Griffith WC, Fenske RA, Kissel JC, et al. Para ninos saludables: a community intervention trial to reduce organophosphate pesticide exposure in children of farmworkers. Environ Health Perspect. 2008;116(5):687–94. doi: 10.1289/ehp.10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffith W, Curl CL, Fenske RA, Lu CA, Vigoren EM, Faustman EM. Organophosphate pesticide metabolite levels in pre-school children in an agricultural community: within- and between-child variability in a longitudinal study. Environ Res. 2011;111(6):751–6. doi: 10.1016/j.envres.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Israel BA, Parker EA, Rowe Z, Salvatore A, Minkler M, Lopez J, et al. Community-based participatory research: lessons learned from the Centers for Children’s Environmental Health and Disease Prevention Research. Environ Health Perspect. 2005;113(10):1463–71. doi: 10.1289/ehp.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.USCB. (ed United States Census Bureau) (2011).
- 17.Coronado GD, Thompson B, Strong L, Griffith WC, Islas I. Agricultural task and exposure to organophosphate pesticides among farmworkers. Environmental Health Perspectives. 2004;112(2):142. doi: 10.1289/ehp.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curl CL, Fenske RA, Kissel JC, Shirai JH, Moate TF, Griffith W, et al. Evaluation of take-home organophosphorus pesticide exposure among agricultural workers and their children. Environ Health Perspect. 2002;110(12):A787–92. doi: 10.1289/ehp.021100787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medlock JM, Leach SA. Effect of climate change on vector-borne disease risk in the UK. The Lancet Infectious Diseases. 2015;15(6):721–30. doi: 10.1016/S1473-3099(15)70091-5. [DOI] [PubMed] [Google Scholar]
- 20.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS-a Bayesian modelling framework: concepts, structure, and extensibility. Statistics and computing. 2000;10(4):325–37. [Google Scholar]
- 21.Lunn D, Jackson C, Best N, Thomas A, Spiegelhalter D. The BUGS book: A practical introduction to Bayesian analysis. CRC press; 2012. [Google Scholar]
- 22.Coronado GD, Vigoren EM, Griffith WC, Faustman EM, Thompson B. Organophosphate Pesticide Exposure Among Pome and Non-Pome Farmworkers: A Subgroup Analysis of a Community Randomized Trial. J Occup Environ Med. 2009;51(4):500–09. doi: 10.1097/JOM.0b013e31819b9ce8. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Driver JH, Li Y, Ross JH, Krieger RI. Dialkylphosphates (DAPs) in fruits and vegetables may confound biomonitoring in organophosphorus insecticide exposure and risk assessment. J Agric Food Chem. 2008;56(22):10638–45. doi: 10.1021/jf8018084. [DOI] [PubMed] [Google Scholar]
- 24.Coronado GD, Thompson B, Strong L, Griffith WC, Islas I. Agricultural task and exposure to organophosphate pesticides among farmworkers. Environ Health Perspect. 2004;112(2):142–7. doi: 10.1289/ehp.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arcury TA, Marín A, Snively BM, Hernández-Pelletier M, Quandt SA. Reducing farmworker residential pesticide exposure: evaluation of a lay health advisor intervention. Health promotion practice. 2009;10(3):447–55. doi: 10.1177/1524839907301409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forster-Cox SC, Mangadu T, Jacquez B, Corona A. The effectiveness of the promotora (community health worker) model of intervention for improving pesticide safety in US/Mexico border homes. Calif J Health Promo. 2007;5:62–75. [Google Scholar]
- 27.Quandt SA, Arcury TA, Austin CK, Cabrera LF. Latino Immigrants: Preventing Occupational Exposure to Pesticides: Using Participatory Research with Latino Farmworkers to Develop an Intervention. Journal of Immigrant Health. 2001;3(2):85–96. doi: 10.1023/A:1009513916713. [DOI] [PubMed] [Google Scholar]
- 28.Quandt SA, Grzywacz JG, Talton JW, Trejo G, Tapia J, D’Agostino RB, et al. Evaluating the effectiveness of a lay health promoter-led, community-based participatory pesticide safety intervention with farmworker families. Health promotion practice. 2013;14(3):425–32. doi: 10.1177/1524839912459652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradman A, Salvatore AL, Boeniger M, Castorina R, Snyder J, Barr DB, et al. Community-based intervention to reduce pesticide exposure to farmworkers and potential take-home exposure to their families. Journal of exposure science & environmental epidemiology. 2009;19(1):79–89. doi: 10.1038/jes.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salvatore AL, Castorina R, Camacho J, Morga N, Lopez J, Nishioka M, et al. Home-based community health worker intervention to reduce pesticide exposures to farmworkers’ children: A randomized-controlled trial. Journal of exposure science & environmental epidemiology. 2015;25(6):608–15. doi: 10.1038/jes.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bello GA, Arora M, Austin C, Horton MK, Wright RO, Gennings C. Extending the Distributed Lag Model framework to handle chemical mixtures. Environ Res. 2017;156:253–64. doi: 10.1016/j.envres.2017.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergen S, Sheppard L, Kaufman JD, Szpiro AA. Multipollutant measurement error in air pollution epidemiology studies arising from predicting exposures with penalized regression splines. Journal of the Royal Statistical Society. Series C, Applied statistics. 2016;65(5):731–53. doi: 10.1111/rssc.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.