Abstract
Objective
An increased neural response to making mistakes has emerged as a potential biomarker of anxiety across development. The error-related negativity is an event-related potential elicited when people make mistakes on simple laboratory-based reaction time tasks that has been associated with risk for anxiety. The present study examined whether the error-related negativity prospectively predicted the first onset of generalized anxiety disorder (GAD) over 1.5 years in adolescent girls.
Methods
The sample included 457 girls between the ages of 13.5 and 15.5 years, with no history of GAD. At baseline, the error-related negativity was measured using a flankers task. Psychiatric history of the adolescent and biological parent were assessed with diagnostic interviews, and the adolescent completed a self-report questionnaire regarding anxiety symptoms. Approximately 1.5 years later, adolescents completed the same interview.
Results
An increased neural response to errors at baseline predicted first-onset GAD over 1.5 years. The error-related negativity was a significant predictor independent of other prominent risk factors, including baseline anxiety and depression symptoms and parental lifetime psychiatric history. Jointly the error-related negativity and social anxiety symptoms provided the greatest power for predicting first-onset GAD.
Conclusions
The current study provides evidence for the utility of the error-related negativity as a biomarker of risk for GAD during a key developmental period.
Keywords: Adolescence, anxiety, biomarkers, event related potential (ERP)
Introduction
Generalized anxiety disorder (GAD) is a prevalent, chronic, and often severe mental illness characterized by excessive worry and hypervigilance. Individuals with GAD frequently over-utilize health care resources and often experience substantial disability in multiple domains (e.g., social, occupational, etc.) across the lifespan (Wittchen, 2002). In light of the economic burden and degree of impairment associated with GAD, this disorder constitutes a significant public health problem. Prospective work suggests that GAD often begins in late childhood or early adolescence (Beesdo, Knappe, & Pine, 2009). Given this, characterizing developmental trajectories that lead to GAD may improve prevention and intervention strategies.
Increasingly, research has begun elucidating the development of core neural systems that underlie clinical anxiety in an effort to map healthy versus anxious trajectories (Pine, 2007). Identifying neural biomarkers that co-occur with anxiety, as well as predict the onset of anxiety may increase our understanding of the etiopathogenesis of clinical anxiety, as well as increase our ability to implement preventative strategies – before symptoms emerge and become impairing. Considering evidence that treatment earlier in the course of development of anxiety disorders results in better long-term functioning (Mancebo et al., 2014), early identification may be particularly important. Additionally, identifying early biomarkers of risk may provide novel targets of treatment for cognitive, behavioral, or pharmacological approaches.
One promising biomarker of GAD is the error-related negativity (Meyer, 2016). The ERN reflects a burst of electrical activity that appears as a sharp negative-going peak in the event-related potential waveform at fronto-central sites and is elicited when people make mistakes on simple laboratory-based reaction time tasks. The ERN has been shown to be increased in anxious individuals in over 40 studies to date (Moser, Moran, Schroder, Donnellan, & Yeung, 2013). Moreover, the ERN seems to be particularly increased in adults with generalized anxiety disorder (GAD; Weinberg, Klein, & Hajcak, 2012; Weinberg, Olvet, & Hajcak, 2010; Xiao et al., 2011). An increased ERN is found in clinically anxious populations, as well as individuals who have undergone successful treatment who no longer meet criteria for a clinical disorder (Hajcak, Franklin, Foa, & Simons, 2008) – suggesting that a potentiated ERN may be an underlying vulnerability marker. Consistent with this proposition, healthy individuals who have first-degree relatives with clinical anxiety are characterized by an increased ERN (Carrasco et al., 2013; Riesel, Endrass, Kaufmann, & Kathmann, 2011). Then ERN appears to be trait-like – demonstrating excellent test-retest reliability for up to 2 years in adults (Weinberg & Hajcak, 2011) and in children and adolescents (Meyer, Bress, & Proudfit, 2014). Additionally, the ERN has been shown to be between 45% and 60% heritable (Anokhin, Golosheykin, & Heath, 2008). Collectively, these findings suggest that an increased ERN may be a heritable biomarker for GAD that may be useful in characterizing developmental trajectories of risk.
Consistent with findings in adults, research in developmental populations has found a potentiated ERN in a heterogeneous group of clinically anxious children and adolescents (Ladouceur, Dahl, Birmaher, Axelson, & Ryan, 2006) and children with OCD (Carrasco et al., 2013; Hajcak et al., 2008; Hanna et al., 2012). Moreover, this relationship appears early in the course of development – children as young as 6 years old with anxiety disorders are characterized by an increased ERN (Meyer et al., 2013).
Source localization studies (Dehaene, Posner, & Don, 1994; Mathalon, Whitfield, & Ford, 2003; van Veen & Carter, 2002), as well as studies that combine ERP and fMRI (Debener et al., 2005), suggest the anterior cingulate cortex (ACC) as the primary generator of the ERN. The ACC is a region of the medial frontal cortex wherein information about threat, punishment, and pain is assimilated to modify behavioral output (Shackman et al., 2011). Indeed, increased error-related ACC activity has also been found among anxious individuals (Fitzgerald et al., 2005; Paulus, Hozack, Frank, & Brown, 2002; Ursu, Stenger, Shear, Jones, & Cameron, 2003), suggesting that both ERP and fMRI methods are capturing overlapping variance in error-related neural activity that is heightened in anxious individuals.
In light of these findings, we have proposed a model that an increased ERN (and error-related ACC activity) among anxious individuals may reflect increased sensitivity to the threat-value of errors. That is, a large ERN may indicate that errors are more aversive for these individuals. Indeed, errors do prompt a series of physiological responses consistent with defensive mobilization (e.g., skin conductance response, heart rate deceleration, potentiated startle reflex, pupil dilation, corrugator muscle contraction, for review, see: Weinberg, Riesel, & Hajcak, 2012). This notion is consistent with data suggesting that punishing errors in the lab potentiates ERN magnitude (Riesel, Weinberg, Endrass, Kathmann, & Hajcak, 2012), and work linking harsh parenting styles to an increased ERN in offspring (Brooker & Buss, 2014; Meyer, Proudfit, et al., 2014). Furthermore, recent work has found that children characterized by a large ERN also exhibit greater potentiation of the startle response in the context of aversive images, but not in the context of neutral or pleasant images (Meyer, Glenn, Kujawa, Klein, & Hajcak, 2016) – further linking the ERN to individual differences in threat-sensitivity.
Previous studies have found that among children high in behavioral inhibition, an increased ERN predicts anxiety symptoms later in development (Lahat et al., 2014; McDermott et al., 2009). Furthermore, we have extended this work and found that children with an elevated ERN are particularly prone to environmentally-induced increases in anxiety symptoms – in a large sample of children who experienced Hurricane Sandy, it was the children who were high in temperamental fear and had an increased ERN who displayed post-hurricane symptom increases (i.e., Hurricane Sandy; Meyer, Danielson, et al., 2016). Important to the validation of the ERN as a biomarker of clinical anxiety, we have also found that an increased ERN in 6 year old children predicts the onset of new anxiety disorders 3 years later, even when controlling for baseline anxiety symptoms (Meyer, Hajcak, Torpey-Newman, Kujawa, & Klein, 2015). Taken together, there is accumulating evidence that an increased ERN early in development confers risk for increases in anxiety prospectively.
Thus far, all prospective studies examining the ERN as a marker of risk have been completed in young children (between the ages of 5 and 7). However, the highest risk period for the onset of many anxiety disorders, including GAD, is in adolescence (Beesdo et al., 2009; Kessler et al., 2005). Therefore, it is critical to examine the ability of the ERN to delineate anxious trajectories during this high-risk period. In the current study, we sought to extend previous work and examine whether an increased ERN would confer risk for new onset GAD during a key risk period (between the ages of 13 – 15) in an independent sample. We focus on females, who are 2–3 times more likely than males to develop an anxiety disorder (Pine, Cohen, Gurley, Brook, & Ma, 1998; Wittchen, Nelson, & Lachner, 1998).
Current study
ERPs were recorded at a baseline assessment while 550 female adolescents performed a flankers task to measure the ERN. Participants with a baseline diagnosis of GAD were excluded from the study. The ability of the ERN to predict the onset of GAD, 1.5 years later, was examined while controlling for other risk variables (baseline anxiety and depression symptoms, as well as parental history of psychopathology). We hypothesized that an enhanced ERN at baseline would predict the onset of GAD. To examine specificity to anxiety, we also planned to investigate whether the ERN predicted new onset depression. To determine whether the ERN had incremental predictive value relative to other psychosocial risk factors, we examined the sensitivity, specificity, and positive and negative predictive value for the development of first-onset GAD.
Methods
Participants
The overall sample was comprised of 550 adolescent females between the ages of 13.5 – 15.5, M = 14.39, SD = 0.63. Overall, 80.5% of the sample was Caucasian and non-Hispanic and 57.8% of parents had a bachelor’s degree. Families were initially recruited to the study via a commercial mailing list, online posts, referrals, and community postings. To participate in the study, adolescents had to be fluent in English and have a biological parent that was able to participate in the study. Individuals were excluded from participation if they had an intellectual disability or lifetime history of depression or dysthymia because one of the main aims of the larger study was to predict first-onset depression. Families were paid for their participation. All participants gave consent/assent before completing the study. The study was approved by the Stony Brook University Institutional Review Board.
Participants were excluded from the present analyses if they had a lifetime history of any clinically-significant subthreshold depression at baseline (i.e., Depression Not Otherwise Specified; n = 34); had a diagnosis of GAD at baseline (n = 21); were missing diagnostic interview or self-report data at baseline or the 1.5 year follow-up assessment (n = 30); did not complete the flankers task, (n = 8), resulting in a final sample of 457 participants.
Measures
Adolescents’ psychiatric history was assessed with the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997). At the Time 2 assessment, approximately 1.5 years later, the K-SADS-PL was re-administered to assess potential changes in diagnostic status. The current study focuses on new-onset cases of GAD (N = 27). To determine reliability of GAD diagnosis, 44 K-SADS-PL interviews were audio recorded and scored by a second rater. The interrater reliability was excellent (kappa = 0.9).
The Structured Clinical Interview for the DSM-IV (SCID) was used to assess parental lifetime history of psychopathology (First et al., 1995). At baseline (Time 1), the SCID was administered to the biological parent that accompanied the adolescent (93.9% mothers). To determine reliability of diagnoses, 25 SCID interviews were audio recorded and scored by a second rater. As previously reported (Nelson, Perlman, Klein, Kotov, & Hajcak, 2016), interrater reliability was acceptable, kappa values ranged from 0.62 [generalized anxiety disorder] to 1.00 [dysthymia].
Adolescent anxiety and depression symptoms were assessed using the expanded Inventory of Depression and Anxiety Symptoms (IDAS-II; Watson et al., 2012). Self-reported symptoms were rated for the past 2 weeks, ranging from 1 (not at all) to 5 (extremely). The IDAS-II is a 99 item, factor-analytically derived, inventory of empirically distinct dimensions of anxiety and depression. The current study focuses on the anxiety subscales: dysphoria, panic, social anxiety, claustrophobia, traumatic intrusions, traumatic avoidance, checking, ordering, and cleaning. Scales were computed as average response to items if no more than 15% of items were skipped.
Procedure
In the current study, we focus on the baseline (Time 1) predictors including: the IDAS-II anxiety and depression symptoms, adolescent diagnoses from the KSADS, parent depression and anxiety diagnoses from the SCID, and error-related neural activity. At Time 2, adolescents completed the KSADS interview again to assess for changes in psychiatric diagnoses.
Flankers task
To measure error-related neural activity, participants completed an arrowhead version of the flankers task (Eriksen & Eriksen, 1974) while EEG was recorded. On each trial, horizontally aligned arrowheads were presented for 200 ms, followed by an intertrial interval (ITI) varying randomly between 2,300 and 2,800 ms. Half of the trails were compatible (“≫≫>” or “≪≪<”) and half were incompatible (“≪>≪” or ≫<≫”); the order of trials was randomly determined. Participants were told to press the right mouse button if the center arrow was facing to the right and to press the left mouse button if the center arrow was facing to the left. After a 30 trial practice block, participants completed 11 blocks of 30 trials (330 trials). Each block was initiated by the participant and participants received feedback based on their performance at the end of each block. If performance was 75% correct or lower, the message “Please try to be more accurate” was displayed; if performance was above 90% correct, the message “Please try to respond faster” was displayed; otherwise the message “You’re doing a great job” was displayed.
Psychophysiological recording, data reduction, and analysis
Continuous EEG recordings were collected using an elastic cap and the ActiveTwo BioSemi system (BioSemi, Amsterdam, Netherlands). Thirty-four electrode sites were used, based on the 10/20 system, in addition to two electrodes on the right and left mastoids. Eye blinks and eye movements (electrooculgram, EOG) were recorded using four facial electrodes: vertical eye movements and blinks were measured via two electrodes place approximately 1 cm above and below the right eye and horizontal eye movements were measured via two electrodes located approximately 1 cm outside the outer edge of the right and left eyes. The EEG signal was pre-amplified at each electrode to improve the signal-to-noise ratio and amplified with a gain of 1x by a BioSemi ActiveTwo system. The data were digitized at 24 bit resolution with a sampling rate of 1024 Hz, using a low-pass fifth order sinc filter with a half-power cutoff of 102.4 Hz. Each active electrode was measured online with respect to a common mode sense (CMS) active electrode producing a monopolar (non-differential) channel. Offline, the data were referenced to the average of the right and left mastoids, and band-passed filtered with low and high cutoffs of 0.1 and 30 Hz, respectively. To detect and reject artifacts, we used an automatic procedure for all segmented data: with a criteria of a voltage step of more than 50.0 μV between sample points, a maximum voltage difference of less than .50 μV within any 100 ms interval, and a voltage difference of 300.0 μV within a trial. These intervals were rejected from individual channels within a trial. Eye-blink and ocular corrections were conducted per Gratton, Coles, and Donchin (1983).
EEG activity was segmented for error and correct responses, 500 ms before the response, continuing 1,000 ms following the response (i.e., 1,500 ms epochs). Error and correct trials were averaged separately. The mean activity in a 200-ms window from −500 to −300 ms prior to the response served as the baseline and was subtracted from each data point. For each participant, the ERN was quantified as the average activity from 0 – 100 ms after error commission, at FCz, where error related activity was maximal. Additionally, the correct response negativity (CRN) was quantified in the same time window at FCz, after correct responses. To isolated error-specific brain activity, analyses focused on the ΔERN - quantified as the ERN minus the CRN.
Behavioral measures include the number of error trials for each participant, as well as reaction time (i.e., RT) on error and correct trials. Post-error RT was calculated as the average reaction time following error trials for each participant. Trials were removed from analyses if reaction times were faster than 200 ms or slower than 1,000 ms.
All statistical analyses were conducted using SPSS (Version 22.0) General Linear Model software, with Greenhouse-Geisser correction applied to p values with multiple-df when necessitated by violation of the assumption of sphericity. Pearson correlation coefficients were used to examine relationships between behavioral data, error-related brain activity, and all anxiety and depression IDAS symptoms. All study variables were standardized for regression analyses. A logistic regression was used to examine whether error-related brain activity predicted the first onset of GAD from Time 1 to Time 2. We also conducted a follow-up simultaneous logistic regression to examine whether the ΔERN uniquely predicted the onset of anxiety disorders when controlling for all significant baseline adolescent anxiety and depression symptoms, as well as parental history of psychopathology.
Additionally, follow-up analyses were conducted in which adolescents with any baseline anxiety disorders were removed from the sample, and a simultaneous logistic regression was again used to examine the unique ability of the ΔERN to predict the first onset of GAD, while controlling for other significant risk factors. To examine the specificity of the relationship between the ΔERN and anxiety, we also conducted a simultaneous logistic regression predicting the onset of depression.
Receiver operating characteristic curve (ROC) analyses were conducted to determine area under the curve, sensitivity, and specificity for baseline predictors in relation to GAD onset between Time 1 and 2. Error-related brain activity and baseline symptoms are continuous measures. To accommodate this, multiple sensitivity and specificity values were calculated based on a range of cutoff scores (+2.0, +1.5, +1.0, and +0.5 SD above the mean). Additionally, values were also calculated when using error-related brain activity and baseline symptoms in parallel, using an “or” and an “and” approach (Weinstein, Obuchowski, & Lieber, 2005). Parallel testing using the “or” approach was calculated using the following formulas: sensitivity = (A)SEN + (B)SEN – [(A)SEN x (B)SEN] and specificity = (A)SPEC x (B)SPEC. Parallel testing using the “and” approach was calculated using the following formulas: sensitivity = (A)SEN x (B)SEN and specificity = (A)SPEC + (B)SPEC – [(A)SPEC x (B)SPEC]. The combined sensitivity and specificity values were then used to calculate the combined positive and negative predictive values.
Results
Behavioral data
Across the sample, accuracy was 86.6%, SD = 5.93. Overall, participants were faster on error trials, M = 357.89 ms, SD = 56.92, than on correct trials, M = 446.79 ms, SD = 63.27, F(1, 444) = 2222.62, p < .001. Consistent with previous studies (Rabbitt, 1966), participants were slower on trials that followed error trials, M = 456.48, SD = 79.19, compared to trials that followed correct trials, M = 431.67, SD = 62.19, F(1, 444) = 186.85, p < .001. Behavioral variables did not correlate with any of the IDAS symptom measures or GAD status.
Error-related brain activity
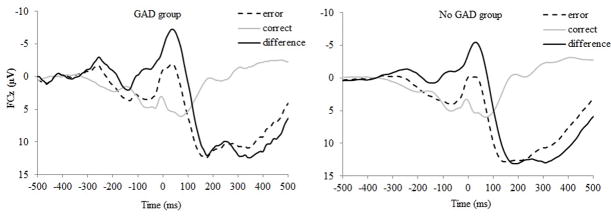
Overall, the ERP response was more negative on error trials, M = 2.53, SD = 8.87, compared to correct trials, M = 5.40, SD = 6.64, F(1, 456) = 88.29, p < .001. At the second assessment, 27 participants (5.9%) experienced first-onset GAD. Average ERP values for the GAD and No GAD groups are presented in Table 1. As can be seen in Figure 1, an increased ΔERN at the baseline assessment predicted an increased likelihood of developing first-onset GAD 18-months later, odds ratio = 0.64, p < .05, 95% CI: 0.42 – 0.99.1,2
Table 1.
Means and standard deviations of clinical variables and error-related brain activity, for children who did (GAD) and did not (No GAD) develop generalized anxiety disorder between the Time 1 and Time 2 assessment.
| GAD (N = 27) | No GAD (N = 430) | |
|---|---|---|
| Time 1 Clinical Variables | ||
| Dysphoria | 2.17 (.89)* | 1.59 (.69)* |
| Panic | 1.62 (.91)* | 1.31 (.52)* |
| Social Anxiety | 2.56 (1.17* | 1.70 (.80)* |
| Claustrophobia | 1.52 (.91) | 1.35 (.70) |
| Traumatic Intrusions | 1.74 (.94)* | 1.36 (.66)* |
| Traumatic Avoidance | 2.17 (.83)* | 1.78 (.96)* |
| Checking | 2.29 (1.02)* | 1.77 (.93)* |
| Ordering | 1.85 (.83) | 1.71 (.77) |
| Cleaning | 1.57 (.58) | 1.44 (.66) |
| Parental Anxiety | 53.85% | 38.61% |
| Parental GAD | 0.03% | 0.08% |
| Paternal Depression | 40.74%* | 23.07%* |
| ERPs (μV) | ||
| ERN | .47 (8.07) | 2.66 (8.91) |
| CRN | 5.67 (6.71) | 5.38 (6.65) |
| ΔERN | −5.20 (6.27)* | −2.72 (6.51)* |
p < .05
Figure 1.
Waveforms are presented for error and correct trials, as well as the difference (error minus correct) at electrode FCz for the GAD group (i.e., adolescents who experienced first-onset GAD; on the left) and the No GAD group (i.e., adolescents who did not experience a first-onset GAD; on the right).
Adolescents who developed GAD by Time 2 were also characterized by increased dysphoria, F(1, 442) = 15.52, p < .001, panic, F(1, 442) = 7.56, p < .01, social anxiety, F(1, 442) = 27.33, p < .01, traumatic intrusions, F(1, 442) = 7.51, p < .01, traumatic avoidance, F(1, 442) = 4.08, p < .05, and checking, F(1, 442) = 7.37, p < .01, symptoms at Time 1 (means and standard deviations for both groups are presented in Table 1). Parental history of any anxiety disorder did not confer risk for the development of GAD in adolescents, χ2(1) = 2.36, p = .12. Additionally, parental history of GAD did not confer risk for the development of GAD in adolescents, χ2(1) = 1.84, p = .17. However, offspring with a parental history of depression were more likely to have GAD by Time 2, χ2(1) = 5.01, p < .05.
To examine the independent ability of these factors to predict increases in anxiety, all significant risk factors were standardized and entered as simultaneous predictors in a logistic regression predicting the onset of GAD. As can be seen in Table 2, results suggested that baseline social anxiety symptoms and the ΔERN uniquely predicted the onset of GAD at Time 2. These results suggest that a potentiated ΔERN prospectively predicts the first onset of a GAD diagnosis independent of other risk factors (i.e., baseline dysphoria and anxiety symptoms, as well as parental history of depression).
Table 2.
Logistic Regression in which Time 1 adolescent depression and anxiety symptoms, parental depression, as well as Time 1 error-related neural activity are entered simultaneously predicting the onset of GAD between the Time 1 and Time 2 assessment.
| New onset GAD | |||
|---|---|---|---|
| B | Wald | OR[95% CI] | |
| Constant | −2.75 | 47.10 | |
| Dysphoria | .35 | 1.11 | 1.42 [.74, 2.73] |
| Panic | −.15 | .27 | .86 [.549 1.52] |
| Social Anxiety | .79 | 8.97 | 2.19 [1.31, 3.66]** |
| Traumatic Intrusions | −.22 | .53 | .81[.45, 1.44] |
| Traumatic Avoidance | .05 | .04 | 1.06 [.64, 1.74] |
| Checking | −.01 | .00 | .99 [.61, 1.61] |
| Parental Depression | −.69 | 2.37 | .50 [.21, 1.21] |
| ΔERN | −.50 | 3.73 | .61 [.37, 1.01]* |
p < .05,
p < .01
Overall model: R2 = .18 (Nagelkerke). Model χ2 = 27.88, p < .01
In the study sample, adolescents were excluded if they met criteria for GAD at Time 1. However, some participants met criteria for other anxiety disorders at Time 1 (obsessive-compulsive disorder, N = 6; panic disorder, N = 4; separation anxiety disorder, N = 9; social anxiety disorder, N = 40; specific phobia, N = 58; anxiety disorder not otherwise specified, N = 24). When we removed adolescents with any anxiety disorder at baseline, 14 adolescents experienced a new-onset GAD at Time 2 and the overall pattern of results remained the same. The ΔERN predicted the onset of GAD, odds ratio = 0.49, p < .05, 95% CI: 0.26 – 0.90.
To examine the specificity of the relationship between the ΔERN and anxiety, we also examined whether the ΔERN would predict the onset of depression. Between the Time 1 and Time 2 assessment, 49 girls experienced a first-onset depressive disorder. However, the ΔERN did not predict the onset of depression, odds ratio = .97, p = .86, 95% CI: .0.72 – 1.32, suggesting its relationship is specific to anxiety.
Table 3 depicts results from the ROC curve analysis, focusing on the two unique predictors that emerged in the simultaneous logistic regression – social anxiety symptoms and the ΔERN. Area under the curve, sensitivity, and specificity values are included for a range of cut-off scores (+2.0, +1.5, +1.0, and +0.5 SD above the mean) for the ΔERN and social anxiety symptoms in predicting the onset of GAD between Time 1 and Time 2. At the values presented, both the ΔERN and social anxiety symptoms provide high specificity, but relatively low sensitivity in predicting GAD status. To examine the additive predictive value of the ΔERN over social anxiety symptoms in predicting the onset of GAD, we examined their combined performance when applied in parallel, in two different ways. First, we examined the predictive ability of parallel testing using an “or” approach – if either test is positive, then the condition is present (Weinstein et al., 2005). In this case, a positive test for social anxiety symptoms or a positive test for the ΔERN would indicate the onset of GAD (i.e., parallel “or”). After this, we examined the predictive ability of parallel testing using an “and” approach – if both tests are positive, then the condition is present (Weinstein et al., 2005). In this case, a positive test for social anxiety and a positive test for the ΔERN would indicate the onset of GAD (i.e., parallel “and”). As can be seen in Table 4, parallel testing using the “or” method increased sensitivity, but decreased specificity, especially when using a low cutoff score for the ΔERN and social anxiety symptoms. There was minimal change in positive and negative predictive value. In contrast, parallel testing using the “and” method increased specificity, but decreased sensitivity, especially when using a high cutoff score for the ΔERN and social anxiety symptoms. Notably, parallel testing using the “and” approach resulted in a significant increase in positive predictive value, such that an individual with a positive screen using this method - i.e., has an ΔERN more than 2.0 SD above the mean (more negative than −15.8 μV) and social anxiety symptoms more than 1.5 SD above the mean (greater than 3.03) – has a 72.0% probability of experiencing the subsequent onset of GAD. At a cutoff of 1.5 SD above the mean, social anxiety symptoms alone has a positive predictive value of 22.5%; thus, the addition of the ΔERN resulted in 3.2-fold increase in predictive power.
Table 3.
Sensitivity, specificity, positive predictive value, and negative predictive value for baseline assessment measures predicting new-onset GAD at the 1.5 year follow-up assessment. The ΔERN was converted to a positive number, such that more positive values indicated enhanced error-related brain activity in order to better compare it with other risk measures. Receiver operating characteristic (ROC) curve analyses were conducted to determine area under the curve, sensitivity, and specificity values. The table includes values for a range of cut-off scores for the continuous ΔERN and social anxiety symptoms. AUC = area under the curve, SEN = sensitivity, SPEC = specificity, PPV = positive predictive value, NPV = negative predictive value.
| Baseline Assessment | AUC | SEN | SPEC | PPV | NPV | |
|---|---|---|---|---|---|---|
| ΔERN | .60 | +2.0 SD | 11.1 | 98.4 | 30.3 | 94.6 |
| +1.5 SD | 11.1 | 95.8 | 14.2 | 94.5 | ||
| +1.0 SD | 22.2 | 86.5 | 9.3 | 94.7 | ||
| +0.5 SD | 40.7 | 70.2 | 7.9 | 95.0 | ||
| Social Anxiety symptoms | .73 | +2.0 SD | 25.9 | 94.8 | 23.8 | 95.3 |
| +1.5 SD | 37.0 | 92.0 | 22.5 | 95.9 | ||
| +1.0 SD | 44.4 | 85.7 | 16.3 | 96.1 | ||
| +0.5 SD | 63.0 | 65.6 | 10.3 | 96.6 |
Table 4.
Sensitivity, specificity, positive predictive value, and negative predictive value for adolescent baseline error-related brain activity and social anxiety symptoms predicting new-onset GAD at the 1.5 year follow-up assessment and applied in parallel using an “or” rule and using an “and” rule. The ΔERN was converted to a positive number, such that more positive values indicated enhanced error-related brain activity in order to better compare it with other risk measures. Receiver operating characteristic (ROC) curve analyses were conducted to determine area under the curve, sensitivity, and specificity values. The table includes values for a range of cut-off scores for the continuous ΔERN and social anxiety symptoms. AUC = area under the curve, SEN = sensitivity, SPEC = specificity, PPV = positive predictive value, NPV = negative predictive value. In parallel testing, using the “or” rule, if either test is positive, then the disease is present. In parallel testing, using the “and” rule, if both tests are positive, then the disease is present.
| Baseline Assessment | Applied in Parallel “or” | Applied in Parallel “and” | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ΔERN | Social Anxiety Symptoms | SEN | SPEC | PPV | NPV | SEN | SPEC | PPV | NPV |
| +2.0 SD | +2.0 SD | 34.2 | 93.3 | 24.2 | 95.8 | 2.8 | 99.9 | 63.7 | 94.3 |
| +2.0 SD | +1.5 SD | 44.0 | 90.5 | 22.5 | 96.3 | 4.1 | 99.9 | 72.0 | 94.3 |
| +2.0 SD | +1.0 SD | 50.6 | 84.3 | 16.8 | 96.5 | 4.9 | 99.8 | 60.6 | 94.4 |
| +2.0 SD | +0.5 SD | 67.1 | 64.6 | 10.6 | 96.9 | 6.9 | 99.4 | 41.9 | 94.4 |
| +1.5 SD | +2.0 SD | 34.1 | 88.1 | 15.2 | 95.5 | 2.9 | 99.7 | 37.7 | 94.3 |
| +1.5 SD | +1.5 SD | 44.0 | 88.2 | 18.9 | 96.2 | 4.1 | 99.7 | 46.1 | 94.3 |
| +1.5 SD | +1.0 SD | 50.6 | 82.1 | 15.1 | 96.4 | 4.9 | 99.4 | 33.9 | 94.3 |
| +1.5 SD | +0.5 SD | 67.1 | 62.8 | 10.2 | 96.8 | 7.0 | 98.6 | 23.9 | 94.4 |
| +1.0 SD | +2.0 SD | 42.4 | 82.0 | 12.9 | 95.8 | 5.7 | 99.3 | 33.8 | 94.4 |
| +1.0 SD | +1.5 SD | 51.0 | 79.6 | 13.6 | 96.3 | 8.2 | 98.9 | 31.9 | 94.5 |
| +1.0 SD | +1.0 SD | 56.7 | 74.1 | 12.1 | 96.5 | 9.9 | 98.1 | 24.6 | 94.6 |
| +1.0 SD | +0.5 SD | 71.2 | 56.7 | 9.3 | 96.9 | 14.0 | 95.4 | 16.0 | 94.7 |
| +0.5 SD | +2.0 SD | 56.1 | 66.5 | 9.5 | 96.0 | 10.5 | 98.5 | 30.5 | 94.6 |
| +0.5 SD | +1.5 SD | 62.6 | 64.6 | 10.0 | 96.5 | 15.1 | 97.6 | 28.3 | 94.8 |
| +0.5 SD | +1.0 SD | 67.0 | 60.2 | 9.5 | 96.7 | 18.1 | 95.7 | 20.9 | 94.9 |
| +0.5 SD | +0.5 SD | 78.1 | 46.1 | 8.3 | 97.1 | 25.6 | 89.7 | 13.5 | 95.1 |
Discussion
The current study is the first demonstration that increased error-related brain activity has clinical utility in predicting the onset of anxiety disorders during a critical risk period (adolescence in females). In a sample of 457 adolescent females with no history of generalized anxiety disorder, increased error-related brain activity predicted first-onset of GAD 1.5 years later. Moreover, the ERN predicted the onset of GAD even when controlling for other better established risk factors – baseline adolescent anxiety and dysphoric symptoms, as well as parental history of psychopathology. Additionally, the ERN provided incremental positive predictive value for new onset GAD when applied in combination with baseline social anxiety symptoms, the only uniquely significant clinical predictor; together, the predictors had an overall positive predictive value of 72.0%. Considering the potential utility of identifying biomarkers of risk during critical risk periods, this is a novel and important extension of previous work linking ERN and risk for anxiety prospectively (Lahat et al., 2014; McDermott et al., 2009; Meyer et al., 2015).
In light of the fact that the ERN continues to be elevated in children with OCD after successful symptom reduction (Hajcak et al., 2008), is increased in healthy relatives of individuals with anxiety (Carrasco et al., 2013; Riesel et al., 2011), is stable across development (Meyer, Bress, et al., 2014), predicts the onset of anxiety disorders in young children (Meyer et al., 2015), and, in the present study, during a critical risk period in adolescent females - the ERN may be considered a developmental biomarker of risk for anxiety. Moreover, in the current study, the predictive power of the ERN demonstrated specificity – delineating anxious versus depressive trajectories, as well as GAD versus other forms of new onset anxiety disorders. Additionally, the ERN has been shown to be heritable (Anokhin et al., 2008), and linked to specific genetic polymorphisms (Manoach & Agam, 2013; Meyer et al., 2012) – making it a potential endophenotype for anxiety disorders (Olvet & Hajcak, 2008).
The current study is the first, to our knowledge, to demonstrate that the ERN can predict the onset of GAD during adolescence and may thus facilitate identification of at-risk individuals in a critical vulnerability period. This finding has implications for the clinical utility of the ERN, such that the ERN is not only useful in early childhood, but also across development, in identifying individuals who are at risk of anxiety disorders. Importantly, the current study examined the use of the ERN, in conjunction with baseline anxiety symptoms, using ROC curve analysis. A decision-making algorithm requiring a positive score on either the ERN or IDAS social anxiety scale had moderate sensitivity (67.1%) and specificity (64.6%) using a cut-off score of +2.0 SD for the ERN and +0.5 SD for social anxiety symptoms. An algorithm using an “and” rule such that girls were counted as positive if they met a threshold of +2.0 SD for the ERN and a threshold of +1.5 SD for social anxiety symptoms was even more effective, exhibiting good positive predictive value (72.0%) and excellent negative predictive value (94.3%). Thus, addition of the ERN to baseline social anxiety symptoms, resulted in significantly increased predictive power, suggesting that this potential biomarker has unique clinical utility. It should be noted, however, that even in this sample, very few adolescents would have actually presented as a positive (having a +2.0 SD for ERN and a threshold of +1.5 SD for social anxiety symptoms). Future work is needed to identify more risk markers that can be utilized to create a more robust risk algorithm.
The current study begins to lay the groundwork for identifying values of the ERN that may indicate risk for anxiety. This is a necessary first step towards the development of an ERN evaluation with clinical application. Furthermore, because the magnitude of the ERN increases across development (Davies, Segalowitz, & Gavin, 2004; Tamnes, Walhovd, Torstveit, Sells, & Fjell, 2013), it will be important for future work to identify age-appropriate norms by which risk can be evaluated. For example, in our previous study, we found that an increased ERN in 6-year old children predicted the onset of anxiety disorders by age 9. Due to developmental changes in the ERN, the cut-off scores that could be derived from that study would not be applicable to an adolescent population. For example, it will be necessary to determine if a 14-year old has an increased ERN relative to other 14 year olds. Thus, the current study is useful in beginning to define clinical cut-off scores for the ERN in adolescents.
In the present study, an increased ERN predicted first onsets of GAD, but did not predict the onset of depressive disorders. Although anxiety and depression are among the most frequently comorbid psychological disorders (Kessler et al., 2005), the ERN appears to differentiate between them. We previously found that among a group of individuals with GAD, individuals with comorbid GAD and MDD, and controls, only the GAD group was characterized by an enhanced ERN (Weinberg, Klein, et al., 2012). Similarly, we found that anxiety, but not depression symptoms, relate to an increased ERN in two separate developmental samples (Bress, Meyer, & Hajcak, 2015; Weinberg et al., 2016). Indeed, other work has found a blunted ERN in clinically depressed children (Ladouceur et al., 2012), as well as children at risk for depression (i.e., children with a maternal history of chronic depression; Meyer, Bress, Hajcak, & Gibb, 2016). Taken together, these findings suggest the ERN may be a viable biomarker that can distinguish anxiety from depression.
Moreover, results from the current study suggest that the ERN predicts new onset GAD, but not any other anxiety disorder. Considering previous work linking the ERN to a range of anxiety disorders in children and adolescents (Meyer, 2017), this finding is surprising. While some previous work suggests the ERN predicts heterogeneous clinical anxiety (Meyer et al., 2015), no work has focused on the developmental period between the ages of 13 – 15. It is possible that increased risk for GAD is particularly linked to the ERN during this developmental period. Future work should explore whether the ERN relates to risk for anxiety differentially across different periods of development.
The current investigation examined one neural risk marker, in the context of baseline symptoms and parental history of psychopathology. Future work could combine the ERN with other measures that may confer risk for anxiety – for example, other EEG measures, along with fMRI measures, genes, life stress, cognitive vulnerabilities, executive functioning, cortisol reactivity, startle potentiation, attentional biases, fear learning, or social functioning to identify constellations of risk markers that may confer the best predictive ability. Once new prospective risk markers are established, the next step is to begin adapting them for clinical use (e.g., standardizing paradigms, shortening tasks, developing norms, examining psychometric properties, developing clinical cut-off scores), with the goal of developing a multi-dimensional assessment with superior diagnostic or predictive power.
Moving beyond diagnostic concerns, an intriguing possibility is that the ERN may serve as a novel target for treatment and prevention efforts. It appears that the ERN is enhanced before symptoms become impairing, thereby providing a potentially malleable target for prevention. Indeed, environmental influences have been found to impact ERN magnitude. For example, in the lab, punishing errors (with a loud noise) potentiates the ERN – an effect that persists for at least a week after punishment ends (Riesel et al., 2012). Similarly, both observed and self-reported harsh parenting relates to an enhanced ERN in offspring – and the magnitude of the ERN mediates the relationship between parenting and anxiety disorders in children (Meyer, Proudfit, et al., 2014). We are currently following up on these findings to determine if intervention strategies focusing on parenting styles may alter children’s ERNs and thereby anxiety symptoms. In addition to this, in one study we found that participants who completed an attention bias modification (ABM) training displayed a reduced ERN (Nelson, Jackson, Amir, & Hajcak, 2015). Ongoing research in a large sample of adolescents is currently underway to determine if multiple ABM training sessions can reduce the ERN and thereby risk for anxiety.
Acknowledgments
This study was supported by National Institute of Mental Health grant R01 MH093479 awarded to R.K. The authors would like to thank their staff and team members who contributed to data collection, especially Molly Gromatsky. The authors have declared that they have no competing or potential conflict of interest.
Footnotes
Recent work suggests that regression-based difference scores may be utilized as an alternative to subtraction-based difference scores (Meyer, Lerner, De Los Reyes, Laird, & Hajcak, 2017). In the current investigation, we focus on a subtraction-based difference score (i.e., the ΔERN). However, the pattern of results is the same when using a regression-based difference score: the residualized ERN predicts new onset GAD, odds ratio = .64, p < .05, 95% CI: 0.42 – 0.96.
Additionally, it should be noted that, at the second assessment, 51 participants experienced first-onset social anxiety disorder, 0 participants experienced first-onset OCD, 15 participants experienced first-onset panic disorder, 64 participants experienced first-onset specific phobia, and 33 experienced first-onset separation anxiety. However, the ΔERN at the baseline assessment did not significantly predict an increased likelihood of developing any of these anxiety disorders, all ps > .10.
Conflict of interest statement: No conflicts declared.
References
- Anokhin AP, Golosheykin S, Heath AC. Heritability of frontal brain function related to action monitoring. Psychophysiology. 2008;45(4):524–534. doi: 10.1111/j.1469-8986.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Knappe S, Pine DS. Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatric Clinics of North America. 2009;32(3):483–524. doi: 10.1016/j.psc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress JN, Meyer A, Hajcak G. Differentiating anxiety and depression in children and adolescents: Evidence from event-related brain potentials. Journal of Clinical Child & Adolescent Psychology. 2015;44(2):238–249. doi: 10.1080/15374416.2013.814544. [DOI] [PubMed] [Google Scholar]
- Brooker RJ, Buss KA. Harsh parenting and fearfulness in toddlerhood interact to predict amplitudes of preschool error-related negativity. Developmental Cognitive Neuroscience. 2014;9:148–159. doi: 10.1016/j.dcn.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Harbin SM, Nienhuis JK, Fitzgerald KD, Gehring WJ, Hanna GL. Increased Error-Related Brain Activity In Youth With Obsessive-Compulsive Disorder And Unaffected Siblingseased Error-Related Brain Activity In Youth With Obsessive-Compulsive Disorder And Unaffected Siblings. Depression and anxiety. 2013;30(1):39–46. doi: 10.1002/da.22035. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7- to 25-year-olds. Developmental neuropsychology. 2004;25(3):355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. The Journal of Neuroscience. 2005;25(50):11730–11737. doi: 10.1523/jneurosci.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Don MT. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5(5):303–305. [Google Scholar]
- Eriksen B, Eriksen C. Effects of noise letters upon the identification of a target letter in a nonsearch task. Attention, Perception, & Psychophysics. 1974;16(1):143–149. doi: 10.3758/bf03203267. [DOI] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, Davies M, Borus J, … Rounsaville B. The structured clinical interview for DSM-III-R personality disorders (SCID-II). Part II: Multi-site test-retest reliability study. Journal of personality disorders. 1995;9(2):92–104. [Google Scholar]
- Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, Taylor SF. Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biological Psychiatry. 2005;57(3):287–294. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and clinical neurophysiology. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Franklin ME, Foa EB, Simons RF. Increased Error-Related Brain Activity in Pediatric Obsessive-Compulsive Disorder Before and After Treatment. Am J Psychiatry. 2008;165(1):116–123. doi: 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- Hanna GL, Carrasco M, Harbin SM, Nienhuis JK, LaRosa CE, Chen P, … Gehring WJ. Error-Related Negativity and Tic History in Pediatric Obsessive-Compulsive Disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2012 doi: 10.1016/j.jaac.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, … Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry. 2006;47(10):1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Slifka JS, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Altered error-related brain activity in youth with major depression. Developmental Cognitive Neuroscience. 2012;2(3):351–362. doi: 10.1016/j.dcn.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat A, Lamm C, Chronis-Tuscano A, Pine DS, Henderson HA, Fox NA. Early behavioral inhibition and increased error monitoring predict later social phobia symptoms in childhood. Journal of the American Academy of Child & Adolescent Psychiatry. 2014 doi: 10.1016/j.jaac.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancebo MC, Boisseau CL, Garnaat S, Eisen JL, Greenberg B, Sibrava NJ, … Rasmussen SA. Long-term Course of Pediatric Obsessive-Compulsive Disorder: Three Years of Prospective Follow-up. Comprehensive Psychiatry. 2014 doi: 10.1016/j.comppsych.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Agam Y. Neural markers of errors as endophenotypes in neuropsychiatric disorders. Frontiers in human neuroscience. 2013:7. doi: 10.3389/fnhum.2013.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Whitfield SL, Ford JM. Anatomy of an error: ERP and fMRI. Biological psychology. 2003;64(1–2):119–141. doi: 10.1016/s0301-0511(03)00105-4. [DOI] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65(5):445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Klein DN, Torpey DC, Kujawa AJ, Hayden EP, Sheikh HI, … Hajcak G. Additive effects of the dopamine D2 receptor and dopamine transporter genes on the error-related negativity in young children. Genes, Brain and Behavior. 2012 doi: 10.1111/j.1601-183X.2012.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A. Developing Psychiatric Biomarkers: a Review Focusing on the Error-Related Negativity as a Biomarker for Anxiety. Current Treatment Options in Psychiatry. 2016:1–9. [Google Scholar]
- Meyer A. A biomarker of anxiety in children and adolescents: a review focusing on the error-related negativity (ERN) and anxiety across development. Developmental Cognitive Neuroscience. 2017;27:58–68. doi: 10.1016/j.dcn.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Bress J, Proudfit GH. Psychometric Properties of the Error-Related Negativity in Children and Adolescents. Psychophysiology. 2014 doi: 10.1111/psyp.12208. [DOI] [PubMed] [Google Scholar]
- Meyer A, Bress JN, Hajcak G, Gibb BE. Maternal Depression Is Related to Reduced Error-Related Brain Activity in Child and Adolescent Offspring. Journal of Clinical Child & Adolescent Psychology. 2016:1–12. doi: 10.1080/15374416.2016.1138405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Danielson CK, Danzig AP, Bhatia V, Bromet E, Carlson G, … Klein D. Neural reactivity to mistakes and temperamental fearfulness prospectively predict the impact of Hurricane Sandy stressors on internalizing symptoms in children. JAACAP 2016 [Google Scholar]
- Meyer A, Glenn CR, Kujawa A, Klein D, Hajcak G. Error-related brain activity is related to aversive potentiation of the startle response in children, but only the ERN is associated with anxiety disorders. Emotion. 2016 doi: 10.1037/emo0000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Torpey-Newman DC, Kujawa A, Klein DN. Enhanced error-related brain activity in children predicts the onset of anxiety disorders between the ages of 6 and 9. Journal of Abnormal Psychology. 2015;124(2):266. doi: 10.1037/abn0000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Torpey DC, Kujawa A, Kim J, Bufferd S, … Klein DN. Increased error-related brain activity in six-year-old children with clinical anxiety. Journal of Abnormal Child Psychology. 2013;41(8):1257–1266. doi: 10.1007/s10802-013-9762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Lerner MD, De Los Reyes A, Laird RD, Hajcak G. Considering ERP difference scores as individual difference measures: Issues with subtraction and alternative approaches. Psychophysiology. 2017;54(1):114–122. doi: 10.1111/psyp.12664. [DOI] [PubMed] [Google Scholar]
- Meyer A, Proudfit GH, Bufferd SJ, Kujawa AJ, Laptook RS, Torpey DC, Klein DN. Self-reported and observed punitive parenting prospectively predicts increased error-related negativity in six-year-old children. Journal of Abnormal Child Psychology. 2014 doi: 10.1007/s10802-014-9918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JS, Moran TP, Schroder HS, Donnellan MB, Yeung N. On the relationship between anxiety and error monitoring: a meta-analysis and conceptual framework. Frontiers in human neuroscience. 2013:7. doi: 10.3389/fnhum.2013.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Jackson F, Amir N, Hajcak G. Single-session attention bias modification and error-related brain activity. Cognitive, Affective, & Behavioral Neuroscience. 2015:1–11. doi: 10.3758/s13415-015-0365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Perlman G, Klein DN, Kotov R, Hajcak G. Blunted neural response to rewards as a prospective predictor of the development of depression in adolescent girls. American Journal of Psychiatry. 2016;173(12):1223–1230. doi: 10.1176/appi.ajp.2016.15121524. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: Toward an endophenotype. Clinical Psychology Review. 2008;28(8):1343–1354. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Frank L, Brown GG. Error rate and outcome predictability affect neural activation in prefrontal cortex and anterior cingulate during decision-making. NeuroImage. 2002;15(4):836–846. doi: 10.1006/nimg.2001.1031. [DOI] [PubMed] [Google Scholar]
- Pine DS. Research review: a neuroscience framework for pediatric anxiety disorders. Journal of Child Psychology & Psychiatry. 2007;48(7):631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55(1):56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Rabbitt P. Error correction time without external error signals. 1966 doi: 10.1038/212438a0. [DOI] [PubMed] [Google Scholar]
- Riesel A, Endrass T, Kaufmann C, Kathmann N. Overactive error-related brain activity as a candidate endophenotype for Obsessive-Compulsive Disorder: Evidence from unaffected first-degree relatives. Am J Psychiatry. 2011;168(3):317–324. doi: 10.1176/appi.ajp.2010.10030416. [DOI] [PubMed] [Google Scholar]
- Riesel A, Weinberg A, Endrass T, Kathmann N, Hajcak G. Punishment has a lasting impact on error-related brain activity. Psychophysiology. 2012;49(2):239–247. doi: 10.1111/j.1469-8986.2011.01298.x. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12(3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Walhovd KB, Torstveit M, Sells VT, Fjell AM. Performance monitoring in children and adolescents: A review of developmental changes in the error-related negativity and brain maturation. Developmental Cognitive Neuroscience. 2013;6(0):1–13. doi: 10.1016/j.dcn.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursu S, Stenger VA, Shear MK, Jones MR, Cameron SC. Overactive action monitoring in Obsessive-Compulsive Disorder: Evidence from functional magnetic resonance imaging. Psychological Science. 2003;14(4):347–353. doi: 10.1111/1467-9280.24411. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology & behavior. 2002;77(4–5):477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Naragon-Gainey K, Koffel E, Chmielewski M, Kotov R, … Ruggero CJ. Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II) Assessment. 2012;19(4):399–420. doi: 10.1177/1073191112449857. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. Longer term test–retest reliability of error-related brain activity. Psychophysiology. 2011;48(10):1420–1425. doi: 10.1111/j.1469-8986.2011.01206.x. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Klein DN, Hajcak G. Increased error-related brain activity distinguishes Generalized Anxiety Disorder with and without comorbid Major Depressive Disorder. Journal of Abnormal Psychology. 2012 doi: 10.1037/a0028270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Meyer A, Hale-Rude E, Perlman G, Kotov R, Klein DN, Hajcak G. Error-related negativity (ERN) and sustained threat: Conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology. 2016;53(3):372–385. doi: 10.1111/psyp.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Olvet DM, Hajcak G. Increased error-related brain activity in generalized anxiety disorder. Biological psychology. 2010;85(3):472–480. doi: 10.1016/j.biopsycho.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Riesel A, Hajcak G. Integrating multiple perspectives on error-related brain activity: The ERN as a neural indicator of trait defensive reactivity. Motivation and Emotion. 2012:1–17. [Google Scholar]
- Weinstein S, Obuchowski NA, Lieber ML. Clinical evaluation of diagnostic tests. American Journal of Roentgenology. 2005;184(1):14–19. doi: 10.2214/ajr.184.1.01840014. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Nelson CB, Lachner G. Prevalence of mental disorders and psychosocial impairments in adolescents and young adults. Psychological medicine. 1998;28(01):109–126. doi: 10.1017/s0033291797005928. [DOI] [PubMed] [Google Scholar]
- Wittchen HU. Generalized anxiety disorder: prevalence, burden, and cost to society. Depression and anxiety. 2002;16(4):162–171. doi: 10.1002/da.10065. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Wang J, Zhang M, Li H, Tang Y, Wang Y, … Fromson JA. Error-related negativity abnormalities in generalized anxiety disorder and obsessive–compulsive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35(1):265–272. doi: 10.1016/j.pnpbp.2010.11.022. [DOI] [PubMed] [Google Scholar]