Fig. 1.
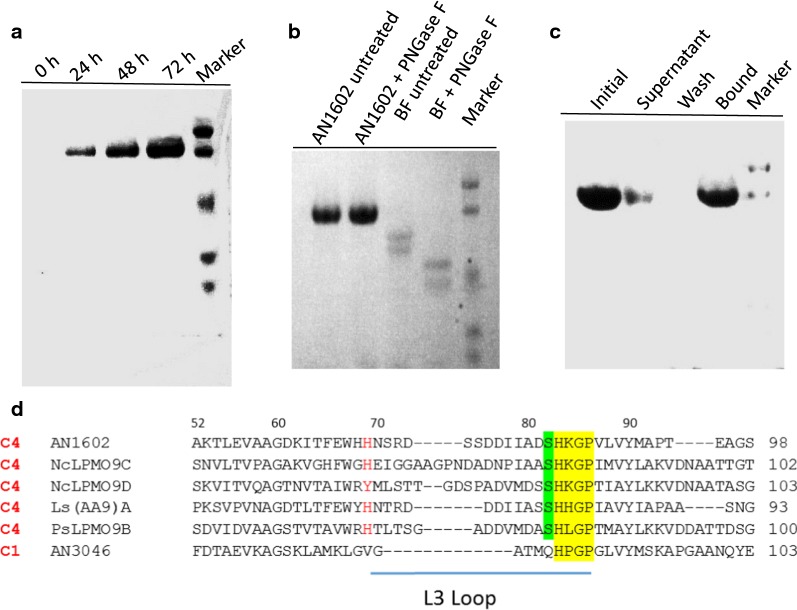
Production of AN1602 in P. pastoris and analysis. a Time course of AN1602 secretion over a period of 3 days from P. pastoris cultures after induction with 1.0% methanol. Aliquots of P. pastoris culture media taken at 24 h intervals were assessed in SDS-PAGE gel under denaturing conditions. b SDS-PAGE analysis of purified AN1602. AN1602 protein was treated with PNGase F and loaded onto a denaturing PAGE gel as follows: purified AN1602 (lane1), AN1602 after PNGase F treatment (lane 2), bovine feutin (BF) (lane 3), BF after PNGase F treatment (lane 4), Marker—low molecular protein standard. Control reactions with BF in lane 3 and lane 4 shows the PNGase F enzyme is effective in deglycosylating BF. c Binding of AN1602 to insoluble cellulose. AN1602 protein was mixed with Avicel and incubated on ice for 3 h in sodium phosphate buffer and the PAGE gel was loaded as fractions containing initial starting material (lane 1), supernatant (lane 2), wash (lane 3) and protein bound to pellet (lane 4). Marker—low molecular protein standard. d Sequence features of AN1602. A partial multiple sequence alignment of selected LPMOs. The conserved [HxnGP] motif containing the second His ligand of the copper active site is highlighted in yellow, and the conserved serine in green. The predicted L3 loop region is marked by a horizontal bar and the first residue of the loop is shown in red. Regioselectivity is (C1/C4) is indicated on the left based on published literature (Frandsen et al. 2016; Isaksen et al. 2014; Jagadeeswaran et al. 2016)