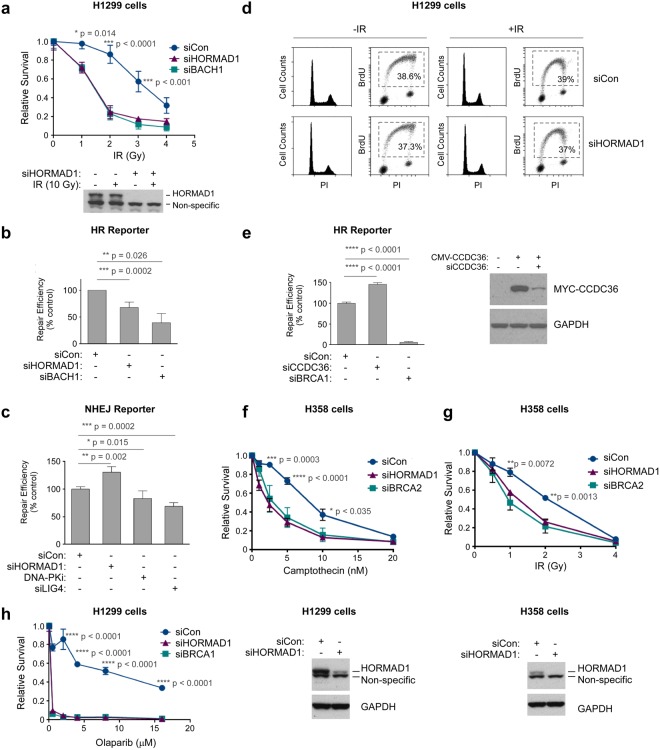
Figure 3.
HORMAD1 promotes Homologous Recombination and DNA damage tolerance in cancer cell lines. (a) Replicate cultures of H1299 cells were transfected with siRNAs targeting HORMAD1 or BRCH1 (or with non-targeting control oligonucleotides). Transfected cells were treated with the indicated dose range of IR and DNA damage sensitivities were measured by clonogenic survival assays. The lower panel is an immunoblot showing relative levels of HORMAD1 expression 48 h post transfection in the siCon- and siHORMAD1-treated cells. (b) Replicate plates of H1299 cells harboring the stably-integrated DR-GFP reporter construct were transfected with siRNAs (against HORMAD1, BACH1, or non-targeting siRNA). 24 h later the siRNA-treated cells were transfected with an ISceI expression plasmid (to induce DSB in the DR-GFP locus) or with an empty control vector. After 24 h cells were trypsinized and GFP-expressing populations (resulting from HR-mediated reconstitution of a silent GFP allele) were enumerated by flow cytometry. Supplementary Fig. S3A shows the original flow cytometry profiles corresponding to these HR assays. In QPCR analyses of mRNA from siBACH1-transfected cells, endogenous BACH1 transcript levels were reduced by 62% relative to control (siCon) cultures. (c) Replicate plates of H1299 cells harboring the stably-integrated EJ5-NHEJ reporter construct were transfected with siRNAs (against HORMAD1, LIG4, or non-targeting siRNA). Some cultures were also treated with the DNA-PK inhibitor NU7441 (20 μM). 24 h later the siRNA or DNA-PKi-treated cells were transfected with an ISceI expression plasmid (to induce DSB in the stably integrated reporter locus) or with an empty control vector. After 24 h cells were trypsinized and GFP-expressing cells (arising from NHEJ-mediated reconstitution of a silent GFP allele) were enumerated by flow cytometry. In QPCR analyses of mRNA from siLIG4-transfected cells, LIG4 transcript levels were reduced by 95% relative to control (siCon) cultures. (d) H1299 cells were transfected with siRNAs targeting HORMAD1 or with non-targeting control oligonucleotides. 48 h later cells were treated with 10 μM BrdU for 1 h to label actively-replicating DNA. Cells were then collected and stained sequentially using a FITC-labeled anti-BrdU antibody and PI. The labeled nuclei were analyzed by flow cytometry. The BrdU-positive (S-phase) populations are indicated by the dashed quadrant. (e) Replicate plates of H1299 cells harboring the stably-integrated DR-GFP reporter construct were transfected with siRNAs against CCDC36, BRCA1, or with non-targeting siRNA (siCon). 24 h later the siRNA-treated cells were transfected with an ISceI expression plasmid and HR activity was measured based on enumeration of GFP-positive cells as described for panel (b) above. In QPCR analyses of mRNA from siBRCA1-transfected cells, BRCA1 transcript levels were reduced by 96% relative to control (siCon) cultures. (f,g) Replicate cultures of H358 cells were transfected with siRNAs targeting HORMAD1 or BRCA2 (or with non-targeting control oligonucleotides). In QPCR analyses of mRNA from siBRCA2-transfected cells, levels of the endogenous BRCA2 transcript were reduced by 92% relative to control (siCon) cultures. Transfected cells were treated with the indicated dose ranges of camptothecin (f) or IR (g) and DNA damage sensitivities were measured by clonogenic survival assays. (h) Replicate cultures of H1299 cells were transfected with siRNAs targeting HORMAD1 or BRCA1 (or with non-targeting control oligonucleotides). Transfected cells were treated with the indicated dose ranges of olaparib and viability was determined by clonogenic survival assays. Error bars throughout this figure represent the standard error of the mean from three independent experiments that were performed in triplicate.