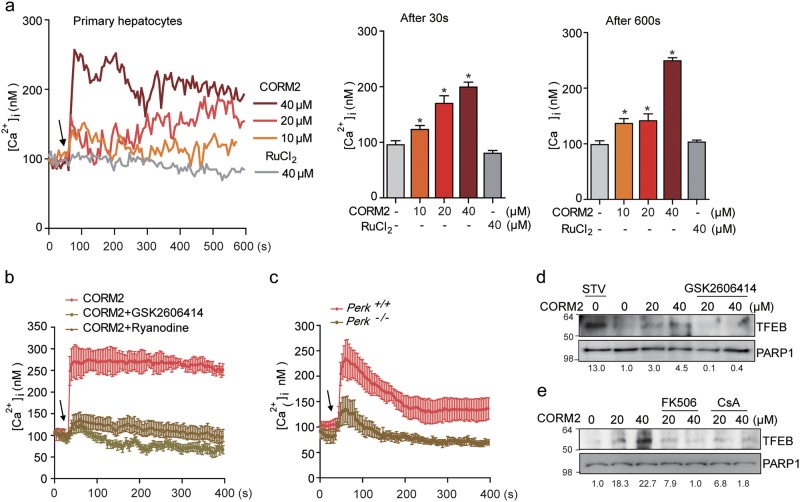
Fig. 2. CO increases TFEB nuclear translocation through PERK-Ca2+-calcineurin pathway.
a Left panel, primary hepatocytes were pretreated with fluo-4 AM and the change of [Ca2+]i was measured using confocal microscopy. The arrow indicates the time point at which CORM2 or ruthenium chloride (control) was added. The data show the mean ± SD from three independent experiments. Middle panel, comparison of mean [Ca2+]i 30 s after treatment with CORM2 or ruthenium chloride. Data are mean ± SD from three independent experiments. *P < 0.001 vs. basal. Right panel, comparison of mean [Ca2+]i 600 s after the treatment with CORM2 or ruthenium chloride. The data are mean ± SD from three independent experiments. *P < 0.001 vs. basal. b GSK2606414 (PERK inhibitor, 1 μM) or Ryanodine (20 μM) was preincubated for 30 min. Arrow indicates the time point at which 30 μM CORM2 was added. Data are mean ± SD from three independent experiments. c CORM2-induced Ca2+ increase was blocked in PERK KO MEF cells. The data show the mean ± SD from three independent experiments. d Primary hepatocytes were pretreated with GSK2606414 for 30 min and then cells were treated with CORM2 or tunicamycin (TM). Endogenous nuclear TFEB was analyzed by immunoblotting. e Primary hepatocytes were incubated with CORM2 or starved for 3 h in the presence or absence of calcineurin inhibitor, FK506 (5 μM), or cyclosporin A (5 μM) for 1 h. Band intensities were determined by densitometry (Image J). Basal levels in the untreated sample were set at 1.0 and results were expressed as fold induction over control levels