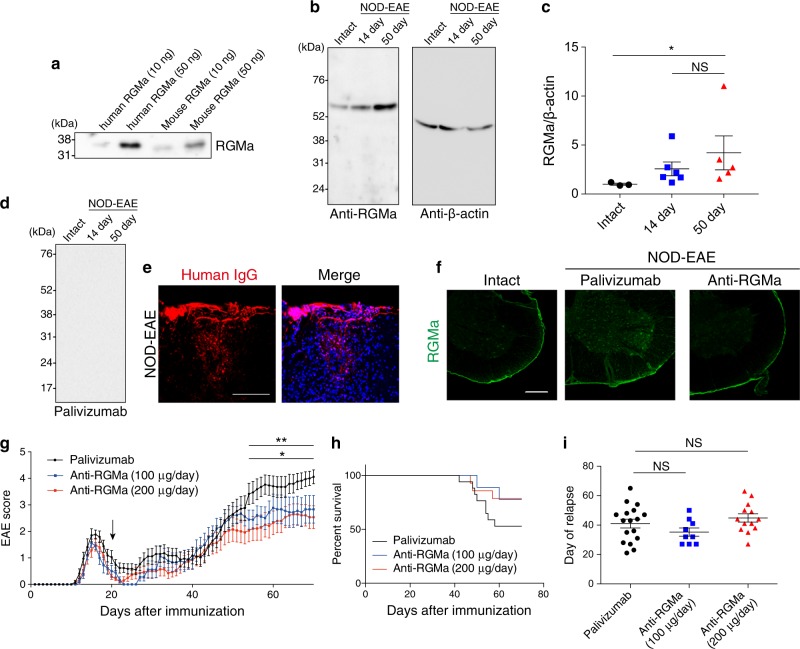
Fig. 1. Treatment with humanized anti-RGMa antibody prevents secondary disease progression in NOD-EAE mice.
a 10 or 50 ng of mouse and human recombinant RGMa proteins were subjected to SDS-PAGE and western blotting with humanized anti-RGMa antibody. b Spinal cord lysates from intact, NOD-EAE mice at 14 days post-immunization and NOD-EAE mice at 50 days post-immunization were subjected to SDS-PAGE and western blotting with humanized anti-RGMa or β-actin antibody. Left numbers show the molecular weight (kDa). c Quantification analysis of RGMa expression in the spinal cord of intact (n = 3), NOD-EAE mice at 14 days post-immunization (n = 6) and NOD-EAE mice at 50 days post-immunization (n = 5). Band intensity of RGMa was normalized to that of β-actin. Statistical analysis was performed by Kruskal−Wallis ANOVA test followed by Dunn’s test. d Spinal cord lysates from intact, NOD-EAE mice at 14 days post-immunization, and 50 days post-immunization were subjected to western blotting with palivizumab. e Representative images show the infiltrating human IgG stained with specific anti-human IgG (red) in the spinal cord of NOD-EAE mice at 70 days after immunization. Counterstaining was performed with DAPI (blue). Scale bar, 50 μm. f Spinal cord sections from control or NOD-EAE mice at 70 days after immunization treated with palivizumab or humanized anti-RGMa antibody were stained with rabbit anti-RGMa antibody. Scale bar: 250 μm. Images are representative of spinal cords extracted from at least three mice per treatment group. g Palivizumab or anti-RGMa antibodies were intravenously injected every 3 days after 20 days of immunization into NOD-EAE mice. EAE clinical scores were determined for NOD-EAE mice treated with palivizumab (200 μg/day, n = 17, black), anti-RGMa (100 μg/day, n = 9, blue) and anti-RGMa (200 μg/day, n = 14, red). The arrow indicates the time (20 days after immunization) when the antibody treatment was started. Statistical analysis was performed by two-way ANOVA followed by Bonferroni tests. *palivizumab vs. anti-RGMa (100 μg/day), **palivizumab vs. anti-RGMa (200 μg/day). (h) Survival curves of NOD-EAE mice treated with palivizumab or anti-RGMa antibody. (i) The days after immunization at which relapse occurred for NOD-EAE mice treated with palivizumab or anti-RGMa. Statistical analysis was performed by one-way ANOVA followed by the Tukey−Kramer test. NS not significant. *p < 0.05, **p < 0.01