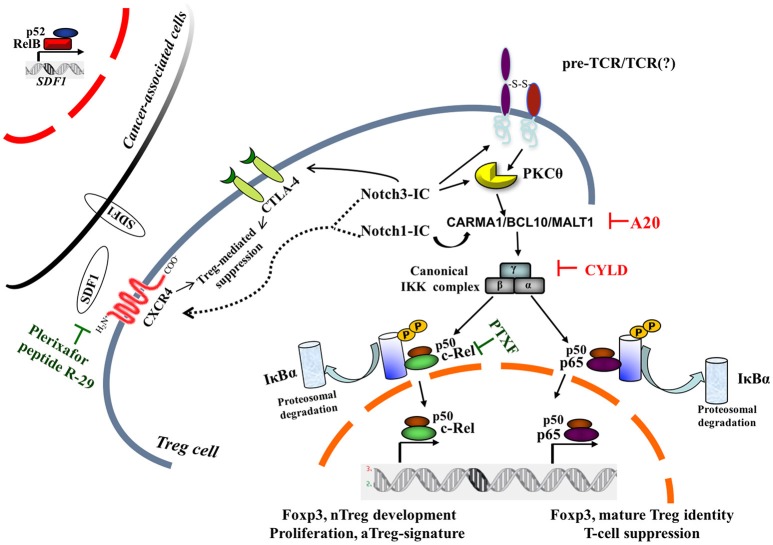
Figure 1.
Canonical NF-κB pathway is central to intrinsic Notch1- and Notch3-modulated Treg cell function within tumor microenvironment. Two NF-κB negative regulators, A20 and CYLD, on removal of nonproteolytic K63-linked polyubiquitin chains from signaling molecules, interfere with the preTCR/TCR pathway, leading to NF-κB activation. For a pharmacological approach, pentoxifylline (PTXF) that selectively degrades c-Rel is indicated, as well as inhibitors of Treg-mediated suppression activity by CXCR4 antagonists, such as plerixafor (AMD3100) or peptide R-29. The dotted line refers to hypothetical Notch1- and/or Notch3-induced CXCR4 modulation in Treg cells, whereas the black curved-line indicates the Notch3-enhanced CTLA4 expression in N3-ICtg Tregs (36). Cancer-associated cells once activated in a tumor microenvironment can express many proinflammatory genes, including stromal cell-derived factor 1 (SDF1), the cognate ligand of CXCR4, partly in an NF-κB-dependent manner (23). pTα-chain (preTCR) and T-cell receptor (TCR); IκBα, inhibitor of NF-κBα.