Figure 2.
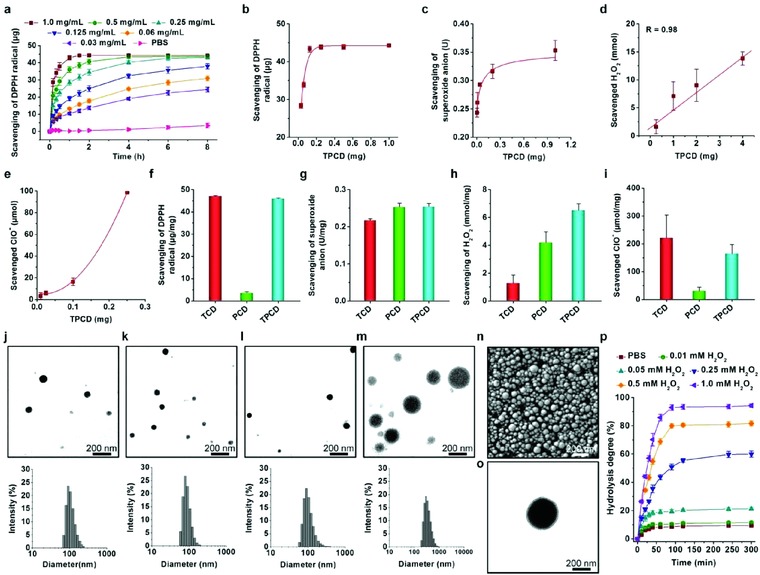
ROS‐scavenging capability of TPCD and characterization of TPCD nanoparticles. a) Time‐dependent scavenging of the DPPH radical by different doses of TPCD. b) DPPH radical‐scavenging efficiency of TPCD at varied doses after 20 h of incubation. c–e) Elimination of superoxide anion, H2O2, and hypochlorite by TPCD after incubation for 40 min, 24 h, and 15 min, respectively. f–i) Comparison of scavenging capabilities of different materials for radical, superoxide anion, H2O2, and hypochlorite. j–m) TEM images (the upper panel) and size distribution profiles (the lower panel) of TPCD NPs fabricated by a self‐assembly/nanoprecipitation method using methanol, methanol/acetonitrile, methanol/dimethylformamide, and methanol/tetrahydrofuran as the solvent for TPCD, respectively. For all solvent mixtures, the volume ratio of methanol to another solvent was kept at 1:2. n) A typical SEM image of TPCD NP prepared using methanol as the solvent. o) TEM image of phosphotungstic acid‐stained TPCD NP. p) Hydrolysis curves of TPCD NP (prepared using methanol as a solvent) in PBS (0.01 m, pH 7.4) containing various concentrations of H2O2. Data in (a–i, p) are mean ± SE (n = 3).
