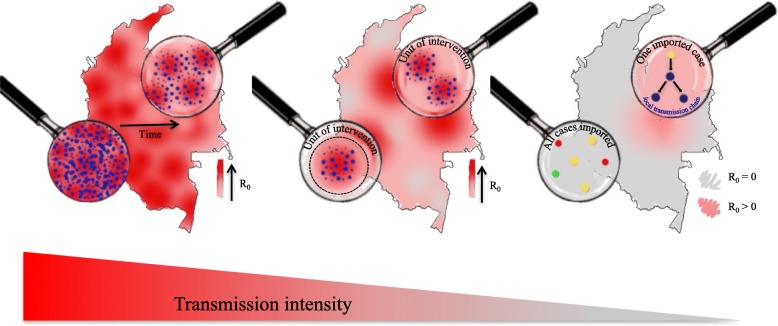
Fig. 1.
Actionable insight from genetic epidemiological studies of malaria across a range of transmission settings. This schematic depicts actionable insight that can be obtained from genetic epidemiological studies of malaria across a range of transmission settings, from high transmission (red) on the left to low transmission (gray) on the right. Here, both imported (stars) and local (points) infections, which may originate from different parasite lineages (various colors), are shown. In high transmission settings, parasites mix panmictically, polyclonal infections are common, and the goal is to evaluate the effectiveness of ongoing interventions. Genetic correlates of declining transmission (e.g., diversity) can provide sensitive indicators of the impact of an intervention. At intermediate transmission, parasites may cluster into interconnected populations. The goal is to delineate regions into units for targeted intervention and to identify the sources that seed transmission for maximally efficient resource allocation. In this setting, models incorporating human mobility and genetic measures of parasite relatedness can provide directional estimates of connectivity between parasite populations. At very low transmission, most infections are imported. The goal is to identify origins of imported parasites, quantify any onward transmission and, if onward transmission exists, the average length of local transmission chains. Models incorporating detailed case data, including genetic data and travel history, can reconstruct transmission chains to infer who acquires infection from who and how