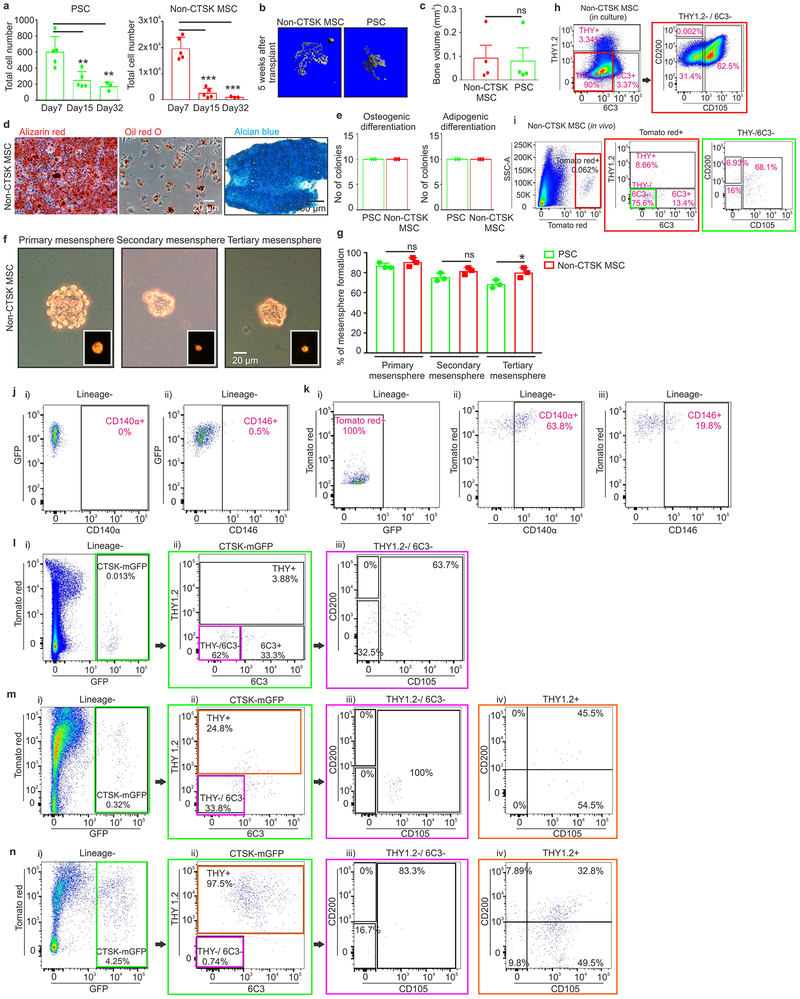
Extended data 3: Functional characterization of non-CTSK MSC cells, PSCs and its derivatives.
a, Total numbers of PSCs and non-CTSK MSCs in mouse femurs at postnatal day 7, day 15 and day 32. Significant decreases in PSCs are observed at day 15 (**p=0.006) and day 32 (**p=0.009) when compared to day 7. Significant decreases in non-CTSK MSCs are observed at day 15 (***p= 3.8×10−5) and day 32 (***p=0.0003) when compared to day 7 (mean ± S.D, n =3 independent experiments; 5 animals/group for day 7, day 15; 3 animals/group for day 32; 2-tailed Student’s t-test). b, μCT images of the bone formed by non-CTSK MSCs (left) and PSCs (right) 5 weeks after transplantation. Representative images from 5 independent experiments. c, Quantification of bone volume (BV) when equal numbers of non-CTSK MSCs and PSCs were transplanted into secondary hosts (p = non-significant (ns), mean ± S.E.M, n= 3 independent experiments, 2-tailed Student’s t-test). d, Clonal non-CTSK MSC colonies were split for differentiation into osteoblasts (Alizarin red staining; left) and adipocytes (Oil red O staining; middle; scale bar 20μm). Separately, chondrocyte differentiation potential was assayed (Alcian blue staining, right; scale bar 100μm). Representative images from 4 independent experiments. e, Clonal differentiation capacity of 10 colonies isolated from PSCs and non-CTSK MSCs after subsequent culture under osteogenic (left) and adipogenic (right) differentiation conditions. All 10 colonies from each population were equally multipotent (mean ± S.D, n= 3 independent experiments. f, Bright field images of primary (left), secondary (middle) and tertiary mesenspheres (right, scale bar 20 μm) derived from non-CTSK MSCs. Tomato red (red) expression is shown in the inserts. Representative images from 3 independent experiments. g, Quantification of % of PSCs and non-CTSK MSCs able to form mesenspheres. *p=0.02, one way ANOVA, Dunnett’s multiple comparison test, mean ± S.D, n = 3 independent experiments. h, FACS analysis of in vitro differentiation of non-CTSK MSCs (right and left plots) after 15 days of culture. Color coded boxes (red) indicates parent/daughter gates. i, FACS plots of non-CTSK MSC-derived cells after the first round of mammary fat pad transplantation (Lineage- = Ter119−, CD45− and CD31−). Color coded boxes (red and green) indicates parent/daughter gates. h-i, Representative FACS plots from 3 independent experiments.
j, FACS for CD140α (i) and CD146 (ii) in PSCs after transplantation into the mammary fat pad. k, FACS for expression of GFP (i),CD140α (ii), and CD146 (iii) in non-CTSK MSCs after mammary fat pad transplantation. l, PP1 cells were transplanted into the mammary fat pad of primary hosts for 2.5 weeks and then analyzed by FACS (i-iii). Color coded boxes (green and magenta) indicate parent/daughter gates. m-n, PP2 cells were isolated by FACS and implanted into the mammary fat-pad of primary recipients. The PP2-derived cells in primary recipients were analyzed by FACS (m, i-iv), and PP2 cells were re-isolated for transplantation into secondary recipients. PP2-derived cells in secondary recipients were analyzed by FACS (n, i-iv). Color coded boxes (green, magenta and orange) indicate parent/daughter gates. j-n, Representative FACS plots from 3 independent experiments.