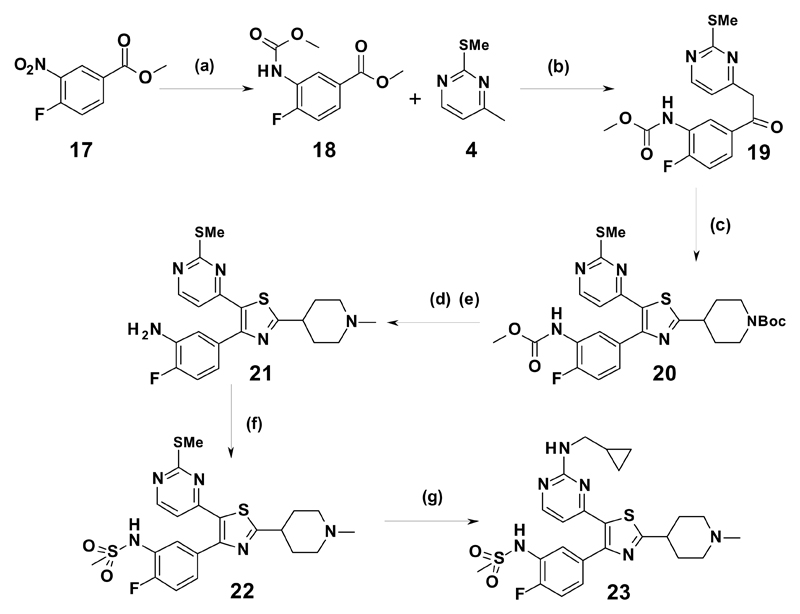
Scheme 3.
Reagents and conditions (a) (i) H-cube, 10% Pd/C, 1 atm, r.t. (ii) methyl chloroformate, pyridine, CH2Cl2, 0 °C to r.t., 94%; (b) LiHMDS (1M in THF), THF, 0 °C to r.t., 67%; (c) (i) (Me)3SiCl, (nBu)4NBr, DMSO, THF, 0 °C to r.t., (ii) tert-butyl 4-carbamothioylpiperidine-1-carboxylate, EtOH, reflux; (d) (i) 4M HCl/dioxane (ii) HCHO, Na(OAc)3BH, AcOH, CH2Cl2; (e) MeSO2Cl, Et3N, CH2Cl2, 0 °C to r.t. 13% from (19); (f) 2M NaOH (aq), dioxane, 80 °C, 49%; (g) (i) H2O2, Na2WO4.2H2O, AcOH; (ii) cyclopropylmethylamine, THF, 70 °C, 11% from (21)