Figure 1. Oncometabolites inhibit α-KG-dependent dioxygenases.
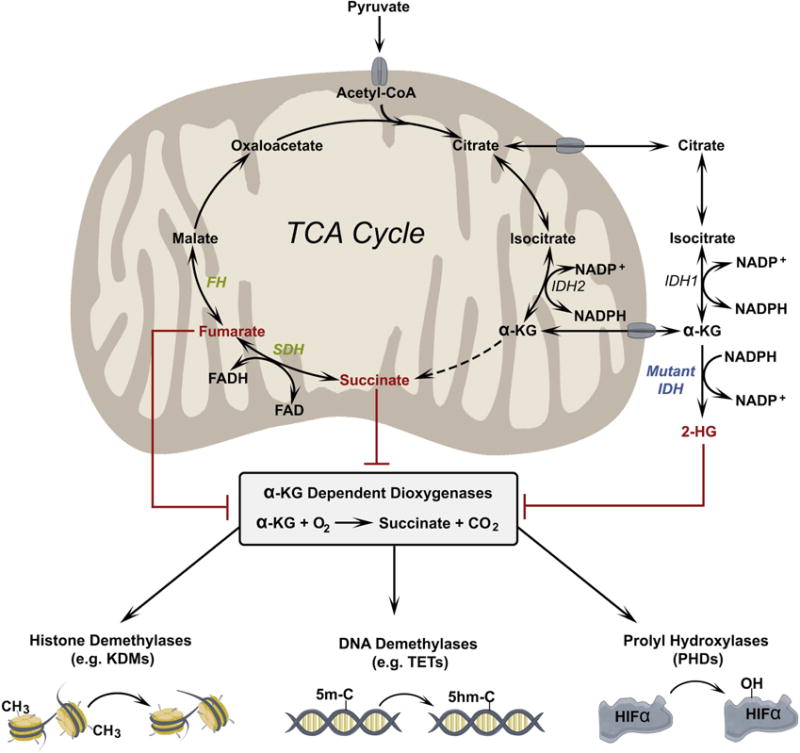
α-KG is required for the function of a family of dioxygenase enzymes including histone demethylases, which remove methyl groups from lysine residues in histone proteins; 5-methylcytosine hydroxylases, which initiate demethylation of cytosine bases; and prolyl hydroxylases, which hydroxylate proline residues in proteins such as the α subunits of hypoxia inducible factors (HIFs). These dioxygenases can be inhibited by high levels of other dicarboxylic acids, which compete with α-KG. Dicarboxylic acids demonstrated to inhibit dioxygenases include D-HG (a product of mutant IDH1/2) and fumarate and succinate, which accumulate due to loss-of-function mutations in FH and SDH, respectively.
