Abstract
The development of medial temporal lobe circuits is critical for subsequent learning and memory functions later in life. The present study reports the expression of progesterone receptor (PR), a powerful transcription factor of the nuclear steroid receptor superfamily, in Cajal-Retzius cells of the molecular layer of the dentate gyrus of rats. PR was transiently expressed from the day of birth through postnatal day 21, but was absent thereafter. Although PR immunoreactive (PRir) cells did not clearly express typical markers of mature neurons, they possessed an ultrastructural morphology consistent with neurons. PRir cells did not express markers for GABAergic neurons, neuronal precursor cells, nor radial glia. However, virtually all PR cells co-expressed the calcium binding protein, calretinin, and the glycoprotein, reelin, both reliable markers for Cajal-Retzius neurons, a transient population of developmentally critical pioneer neurons that guide synaptogenesis of perforant path afferents and histogenesis of the dentate gyrus. Indeed, inhibition of PR activity during the first two weeks of life impaired adult performance on both the novel object recognition and object placement memory tasks, two behavioral tasks hypothesized to describe facets of episodic-like memory in rodents. These findings suggest that PR plays an unexplored and important role in the development of hippocampal circuitry and adult memory function.
Keywords: molecular layer, calretinin, reelin, hippocampus, RRID: AB_90764, RRID: AB_2278725, RRID: AB _94856, RRID: AB_94922, RRID: AB_2298772, RRID: AB_2315192, RRID: AB_2285132, RRID: AB_477627, RRID: SCR_003073
Graphical Abstract
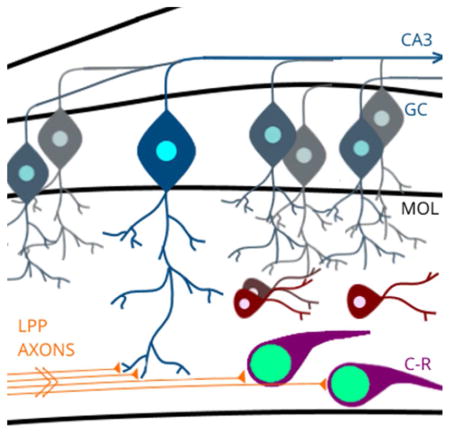
Cajal-Retzius (C-R) cells are pioneer neurons that guide synaptogenesis of lateral perforant path (LPP) axons onto granule cell (GC) dendrites within the developing molecular layer (MOL). The nuclear transcription factor, progesterone receptor (PR), is expressed within C-R cells and may alter the development of hippocampal circuitry. Indeed, inhibition of PR activity during development impaired performance on memory tasks in adulthood.
INTRODUCTION
Neural development is profoundly influenced by steroid hormones which are secreted by peripheral endocrine glands and/or synthesized de novo within the brain. The sensitivity of any brain region to a steroid hormone is conferred by the expression of cognate nuclear receptors which, as powerful transcription factors, regulate fundamental processes of brain maturation including plasticity, synaptogenesis, cell death and expression of neurotrophic factors and their receptors (e.g. Carbone and Handa, 2013; Cooke and Woolley, 2005; Forger, 2006; Garcia-Segura and Melcangi, 2006; Simerly, 2002). Through these mechanisms, nuclear steroid receptors can exert permanent effects on brain structure, function, and subsequent adult behavior. Progesterone receptor (PR) is transiently expressed in many regions of the forebrain, midbrain, and hindbrain during pre- and postnatal development (Quadros, Pfau, & Wagner, 2007; Quadros, Schlueter, & Wagner, 2008), suggesting that progesterone and PR may play a key role in the normal development of the brain, not only in regions involved in neuroendocrine and reproductive function, but also in areas more closely associated with cognition. For example, recent evidence demonstrates that inhibition of PR activity during perinatal life alters the development of the mesocortical dopaminergic pathway and impairs medial prefrontal cortex-dependent behaviors such as cognitive flexibility and behavioral inhibition, in adulthood (Willing & Wagner, 2016), suggesting that PR is involved in establishing proper connectivity of this pathway.
Interestingly, PR is transiently expressed during postnatal life within the hippocampus, a structure critical for learning, spatial memory, and integration of associative recognition for objects, places, and contexts (Eichenbaum & Cohen, 2014; Fortin, Wright, & Eichenbaum, 2004; Hartley, Lever, Burgess, & O’Keefe, 2014; Langston & Wood, 2010; Morris, Garrud, Rawlins, & O’Keefe, 1982; Moser, Moser, & Andersen, 1993). Indeed, previous findings suggest that perinatal exposure to high levels of progesterone impaired maze learning in adult male rats (Hull, Franz, Snyder, & Nishita, 1980), suggesting that PR activity may be involved in the development of hippocampal circuitry.
Within the hippocampus, PR is expressed in the dentate gyrus during perinatal life from approximately embryonic day 22 to postnatal day 14 (E22-P14) (Quadros et al., 2007). Based on observations by Quadros et al., (2007), it appears that nuclear PR immunoreactivity is specifically confined to the molecular layer (MOL) of the dentate gyrus. The MOL is the primary site of input from the perforant path into the dentate gyrus and consists largely of synaptic connections between entorhinal terminals, and granule cell, mossy cell, and hilar interneuron dendrites. As such, cells within the MOL are uniquely poised to modulate incoming signals from sensory afferents at the synaptic interface of perforant path axons and neurons of the dentate gyrus (Amaral, Scharfman, & Lavenex, 2007; Houser, 2007). For instance, in adult rats GABAergic interneurons of the MOL, such as molecular layer perforant path associated cells, neurogliaform cells, and outer molecular layer cells, provide inhibitory modulation via efferent connections to granule cells, CA1 pyramidal cells, and subicular cells respectively, reflecting their complex interaction with both input and output regions of the hippocampal circuit (Armstrong, Szabadics, Tamas, & Soltesz, 2012; Ceranik et al., 1997; Houser, 2007; Li et al., 2013; Quattrocolo & Maccaferri, 2013). Importantly, the timing of PR expression within the MOL coincides with critical developmental processes in the formation of the perforant path connections into this structure, including the arrival of entorhinal cortex terminals (P0-P2) (Deng, Yu, Wu, & Li, 2007; Supèr, Martínez, Del Río, & Soriano, 1998) and initial synaptogenesis within the MOL (P2-P5) (Soriano, Del Río, Martinez, & Supèr, 1994). PR expression in MOL cells during postnatal life may be critical to the proper formation of the developmental trajectory of hippocampal circuitry.
Currently, very little is understood about steroid hormone influence on the development of the hippocampus, and the role of steroid receptor activity on the development of the dentate gyrus and MOL is virtually unstudied. Therefore, the present study investigated the cellular phenotype of PR expressing cells of the perinatal MOL through the use of immunocytochemical and electron microscopic approaches, and examined the potential role of PR activity during development on adult learning and memory. Results demonstrate that PR is expressed within a developmentally critical cell population of the MOL that may be imperative for subsequent recognition memory of objects and their location.
MATERIALS AND METHODS
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University at Albany, SUNY and were in accordance with the 2011 Eighth Edition of the National Institute of Health Guide for the Care and Use of Laboratory Animals. Pregnant female Sprague-Dawley rats were purchased from Taconic Laboratories (Germantown, NY) at gestational day 16 and allowed to deliver pups normally. Male rat pups were used for all experiments. The day of birth was designated postnatal day 1 (P1). All animals were housed on a 12-hour light/12-hour dark cycle at a constant temperature of 25 ± 2°C with food and water ad libitum. Unless otherwise indicated, P7 rats were deeply anesthetized by hypothermia and euthanized by decapitation prior to brain removal and fixation.
Antibody Characterization
All antibodies and antisera used in these experiments have been previously characterized and appropriate controls for specificity performed and published. Primary antibodies used in these experiments are summarized in Table 1.
Table 1.
Primary antibodies and antisera used for immunocytochemistry in this study
| Antibody | Species/Immunogen | Identification | Concentration |
|---|---|---|---|
|
| |||
| Calretinin | Goat polyclonal | RRID: AB_90764 | 1:1000 |
| Purified calretinin from rat | Millipore, Temecula,CA | ||
| Cat #: AB1550 | |||
|
| |||
| GAD67 | mouse monoclonal recombinant fusion protein amino acids 4-101 | RRID: AB_2278725 | 1:2000 |
| Millipore, Temecula, CA | |||
| Cat #: MAB5406 | |||
|
| |||
| MAP2 | Mouse monoclonal | RRID: AB_94856 | 1:1000 |
| MAP2a&b purified from bovine brain | Millipore, Temecula,CA | ||
| Cat #: MAB3418 | |||
|
| |||
| Nestin | Mouse monoclonal | RRID: AB_94911 | 1:400 |
| Nestin purified from embryonic rat spinal cord | Millipore, Temecula, CA | ||
| Cat #: MAB353 | |||
|
| |||
| NeuN | Mouse monoclonal | RRID: AB_2298772 | 1:1000 |
| Purified cell nuclei from mouse brain | Millipore, Temecula, CA | ||
| Cat #: MAB377 | |||
|
| |||
| PR | Rabbit polyclonal | RRID: AB_2315192 | 1:1000 |
| Synthetic peptide corresponding to amino acids 533–547 of human PR-A and B | DAKO, Glostrup, Denmark | ||
| Cat #: A0098 | |||
|
| |||
| Reelin | Mouse monoclonal | RRID: AB_2285132 | 1:2000 |
| Recombinant reelin amino acids 40-18; epitope amino acids between 164–189 | Millipore, Temecula, CA | ||
| Cat #: MAB5366 | |||
|
| |||
| Vimentin | Mouse monoclonal | RRID: AB_477627 | 1:400 |
| Vimentin purified from pig eye lens | Sigma, St. Louis, MO | ||
| Cat #: V6630 | |||
Progesterone receptor rabbit polyclonal
PR antiserum (RRID: AB_2315192; DAKO, Glostrup, Denmark) is a rabbit polyclonal antibody raised against the following sequence of the PR protein, amino acids 533–547 (NYLRPDSEASQSQY), which is the DNA binding domain of the human PR and is contained in both A and B isoforms of the receptor (Traish & Wotiz, 1990). Antibody specificity was tested as described in (López & Wagner, 2009; Quadros, Pfau, Goldstein, De Vries, & Wagner, 2002; Quadros et al., 2007). We have demonstrated that nuclear immunoreactivity was completely eliminated in all regions, including hippocampus, by preadsorption of the PR antiserum overnight at 4°C with either the antigen peptide (Genosys Biotechnologies, The Woodlands, TX) or a 10-fold molar excess of human PR-A and PR-B proteins (Quadros et al., 2002; Tetel et al., 1997); (Tissue Culture CORE Facility, University of Colorado Cancer Center, Denver, CO), or when the primary antiserum was omitted. Additionally, we have demonstrated that all immunoreactivity with this antiserum is abolished in transgenic mice that possess an insertional mutation in the PR gene (i.e., progesterone receptor knockout mice; (Ismail, Li, DeMayo, O’Malley, & Lydon, 2002). Additionally, following preadsorption of the antibody with 150Og/ml of the immunizing peptide overnight at 4°C, nuclear PRir was not detectable in the hippocampus by electron microscopy (Waters, Torres-Reveron, McEwen, & Milner, 2008).
MAP-2 mouse monoclonal (mature neuronal marker)
Microtubule associated protein-2 (MAP-2) is a high molecular weight protein important for brain microtubule assembly as well as a stringent marker for mature neurons (Izant & McIntosh, 1980). The MAP-2 antibody (RRID: AB_94856; Millipore, Temecula, CA; Cat. No. MAB3418; clone AP20, lot no. 2452494) is a mouse monoclonal antibody directed against microtubule protein purified bovine brain that recognizes MAP-2a and b isoforms. In immunoblots done using homogenized cortex samples from postnatal rats, the MAP-2 antiserum produced only one appropriately sized band (~240 kDa; data not shown) specific to MAP-2b, the isoform expressed in developing cortex (Binder et al., 1984; Caceres et al., 1984). MAP-2 immunostaining of adult and developing brain has been well characterized and shown to be abolished by adsorption with a molar excess of the antigen (Caceres et al., 1984).
GAD67 mouse monocolonal (GABAergic interneuron marker)
The enzyme, glutamic acid decarboxylase (GAD67) converts glutamic acid to gamma-aminobutyric acid (GABA), the maor inhibitory neurotransmitter in the brain. GAD67 is specifically expressed in the cell body of neurons and is the main source of cytoplasmic GABA (Kaufman, Houser, Tobin, 1991). The GAD67 monoclonal antibody (clone 1G10.2; RRID: AB_2278725; Millipore, Temecula, CA, Cat. No. MAB5406) is directed against recombinant fusion protein of the N terminal region (amino acids 4–101) of human GAD67. This amino acid sequence is not shared with GAD65. This antibody reacts with mouse, rat and human GAD67 (manufacturer’s datasheet). Western blot analysis with this antibody demonstrates a single band of 67 kDa, with no detectable cross reactivity with GAD65 (Stranahan, Jiam, Spiegel, & Gallagher, 2012). The antibody produces specific staining of GAD67 neurons in rat brain (Stranahan, et al, 2012).
NeuN mouse monoclonal (mature neuronal marker)
NeuN is a DNA-binding nuclear protein and is a well-established marker of terminally differentiated neurons (Mullen, Buck, & Smith, 1992). The NeuN antibody (RRID: AB_2298772; Millipore, Temecula, CA; Cat. No. MAB377) is a mouse monoclonal antibody raised against purified cell nuclei from mouse brain that stains the nuclei of most mature neuronal cell types in the mammalian brain (Mullen et al., 1992). In Western blots, the NeuN antibody detects two or three bands in the molecular weight range 46–48 kDa, representing multiple phosphorylated forms of the same antigen (Lind, Franken, Kappler, Jankowski, & Schilling, 2005).
Vimentin mouse monoclonal (radial glial cell marker)
Vimentin is the major cytoskeletal component in immature glia and is an established marker of transient radial glial cells in the developing CNS (Pixley & de Vellis, 1984). The vimentin mouse monoclonal antibody (RRID: AB_477627; Millipore, Temecula, CA; Cat. No. V6630) is directed against vimentin from pig eye lens (Osborn, Debus, & Weber, 1984) and in Western blots, this antibody detects a single band (58 kDa), and does not detect other closely related intermediate filament proteins including desmin and GFAP (Osborn et al., 1984).
Nestin mouse monoclonal (neural precursor cells)
Nestin is an intermediate filament protein expressed in neural precursor cells and serves as a marker of undifferentiated CNS cells just prior to exit from the cell cycle (Lendahl, Zimmerman, & McKay, 1990; Suzuki, Namiki, Shibata, Mastuzaki, & Okano, 2010). The nestin mouse monoclonal antibody (RRID: AB_94911; Millipore, Temecula, CA; Cat. No. MAB353) is directed against nestin purified from embryonic rat spinal cord. In Western blot analysis, the antibody detects one band in molecular weight range 200–220 kDa. (Hockfield & McKay, 1985).
Calretinin goat polyclonal (interneuron marker)
Calretinin is an intracellular calcium binding protein and can be used as a marker for a subset of interneurons in the hippocampus (Gulyás, Hájos, & Freund, 1996; McBain & Fisahn, 2001) as well as Cajal-Retzius neurons in both the hippocampus and neocortex (Del Rio, Martinez, Fonseca, Auladell, & Soriano, 1995; Jiang & Swann, 1997). The calretinin goat polyclonal antibody (RRID: AB_90764; Millipore, Temecula, CA; Cat. No. AB1550) is raised against rat calretinin. In Western blot analysis the antibody detects one band (31 kDa). Preadsorption of the antibody with recombinant calretinin eliminates immunoreactivity in brain tissue (Winsky, Nakata, Martin, & Jacobowitz, 1989).
Reelin mouse monoclonal (Cajal-Retzius cell marker)
Reelin is an extracellular matrix protein expressed in some of the earliest generated neurons including Cajal-Retzius cells (D’Arcangelo et al., 1995; Hirotsune et al., 1995; Ogawa et al., 1995). The reelin mouse monoclonal antibody (RRID: AB_2285132; Millipore, Temecula, CA; Cat. No. MAB5366) is raised against recombinant reelin amino acids 40–189 with the epitope consisting of amino acids between 164 and 189. In Western blots, the antibody detects three bands: one full length (400–450kDa) and two additional fragments (300 and 180–200 kDa) (Zhao, Chai, Förster, & Frotscher, 2004) MAB3566 produces selective immunohistochemical staining of Cajal-Retzius cells (de Bergeyck, Naerhuyzen, Goffinet, & Lambert de Rouvroit, 1998).
Ontogeny of PR-ir in the Dentate Gyrus
Tissue Preparation
On the day of birth (P1) male pups (n=7) were anesthetized by hypothermia. On P7 (n=7), P10 (n=8), P14 (n=5), P21 (n=6) and P28 (n=4) male pups were anesthetized with a lethal dose of chloryl-hydrate pentobarbital (0.25M chloral hydrate, 0.08M magnesium sulfate, 45mM pentobarbital, 3M ethyl alcohol, 4.5M propylene glycol, i.p.) and intracardially perfused with 20–50ml of 0.9% saline followed by 50–150 ml 5% acrolein in 0.1M phosphate buffer (PB, pH 7.6). Brains were removed from the skull and postfixed in 5% acrolein in 0.1M PB for ~6hr followed by 30% sucrose in 0.1M PB for at least 24 hours prior to sectioning. Sections were cut on a sliding microtome in the coronal plane at 50Om and stored in cryoprotectant (30% sucrose, 0.1% polyvinylpyrrolidone-40, and 30% ethylene glycol in 0.1M PB) at −20°C until immunocytochemical processing.
PR Immunocytochemistry
Immunocytochemistry was performed on free-floating sections using the rabbit polyclonal PR antibody (DAKO Corp.) described above. All incubations were performed at room temperature unless otherwise indicated. Sections were rinsed in Tris-buffered saline (TBS, pH 7.6, 3 x 5 min) to remove residual cryoprotectant solution. Sections were incubated in 1% sodium borohydride in TBS for 10 min, rinsed in TBS (4 x 5 min), then incubated in TBS containing 20% normal goat serum (NGS), 1% H2O2, and 1% bovine serum albumin for 30 min. PR antiserum was diluted 1:1000 in TBS containing 2% NGS, 0.3% Triton X-100, and 0.02% sodium azide (TTG) for 72 hours at 4°C. Following rinses (3 x 5 min) in TTG, sections were incubated for 60 min in biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA) at a concentration of 5 Og/mL in TTG. After two TTG rinses (5 min each) and two TBS rinses (5 min each) sections were incubated in the ABC reagent (Vectastain Elite Kit, Vector Laboratories) for 60 min. Following TBS rinses (3 x 5 min each) sections were incubated in TBS containing 0.05% 3,3’diaminobenzidine, 0.75 mM nickel ammonium sulfate, 0.15% -β-D-glucose, 0.04% ammonium chloride, and 0.001% glucose oxidase for 20 min. After three TBS rinses (5 min each), sections were mounted on gelatin-coated slides before being dehydrated, delipidated, and coverslipped with Permount (Fisher Scientific, Pittsburgh, PA).
Analysis of PR-ir Cell Number
A section through the dorsal hippocampus was selected for each animal and anatomically matched across groups using two prenatal (Paxinos et al., 1994; Altman and Bayer 1995) and one adult (Paxinos and Watson 1998) atlas. The total number of PR-ir nuclei (dark brown or intense black) within the supra and infra-pyramidal blades of the dentate gyrus were counted unilaterally in each representative section at each age. Raw cell counts were corrected using the Abercrombie correction method as previously published by our laboratory (Jahagirdar & Wagner, 2010; Quadros et al., 2007). Briefly, raw cell counts for each animal at each age were multiplied by the correction factor determined by using the formula T/ T + h, where T = section thickness and h = mean nuclear diameter. For each age, mean nuclear diameter was determined using 3 randomly selected animals. From each animal, the diameter of 5 randomly selected PR-ir nuclei was measured from each hemisphere using NIH Image analysis software (https://imagej.nih.gov/nih-image/ RRID: SCR_003073 , for a total of 30 nuclei, to generate an average nuclear diameter for each age group. Using one-way analysis of variance (ANOVA), it was determined that there were no significant differences in the size of counted PR-ir nuclei across ages. The numbers of PR-ir cells (corrected cell counts) were then compared across ages using one-way ANOVA. Pre-planned post hoc comparisons between consecutive ages were conducted using Student Newman-Keuls (P < 0.05).
Colocalization of PR-ir with GAD67, a Marker for GABAergic Interneurons
Tissue Preparation
On P7, pups were anesthetized with a lethal dose of sodium pentobarbital (i.p.) and intracardially perfused with 0.9% saline followed by 50ml 3.75% acrolein and 2% paraformaldehyde in 0.1M phosphate buffer (PB, pH 7.4). Brains were removed from the skull and postfixed in 2% paraformaldehyde in 0.1M PB for 24 hours. Sections were cut at 50μm and stored in cryoprotectant as described above until immunocytochemical processing.
Double Label Fluorescent Immunocytochemistry
For fluorescent double labeling, immunocytochemistry was performed as described above with the following modifications. Tissue was incubated in 1% sodium borohydride in TBS) for 10 min, before blocking in 1% H2O2, 1% bovine serum albumin (BSA), and 20% NGS in TBS buffer (TTG) for 30 min. Sections were then incubated in mouse anti-GAD67 (1:2000) (refer to Table 1) in TBS containing Triton X-100, and 2% NGS for 24 hours at 4°C. After primary incubation, tissue was incubated in fluorescently tagged secondary antibody, AlexaFluor 568 (1:200, goat anti-mouse, Invitrogen Inc.) in TTG buffer for 90 min at room temperature. Tissue was then rinsed for 1 hour in TBS before primary incubation in antisera against PR (1:1000, rabbit polyclonal, DAKO Corp.) in TTG buffer for 72 hours at 4°C as described above. For adequate fluorescence visualization of PR, the immunohistochemical signal was enhanced using the Tyramide Signal Amplification (TSA) Biotin system (PerkinElmer, Inc.). After PR primary incubation, the tissue was incubated in biotinylated secondary antibody (5Og/ml, goat anti-rabbit, Vector Labs) in TTG buffer for 90 min at room temperature, followed by incubation in streptavidin-horseradish peroxidase (SA-HRP, 1:100) in TBS containing 0.3% Triton-X (TTX) for 60 min, and then incubation in Biotinyl Tyramide for 10 min in TTX buffer. Finally, tissue was incubated in a solution containing streptavidin conjugated fluorophore to detect PR (1:1000, AlexaFluor 488, Invitrogen Inc.) in TBS buffer for 60 min at room temperature. Sections were then mounted on slides and coverslipped with ProLong Gold Antifade reagent (Invitrogen Inc.).
Confocal Microscopy
All images were acquired using a Zeiss LSM710 laser scanning confocal microscope attached to a Zeiss Axio Observer Z1 inverted microscope stand. Each image was taken sequentially using a multiline Argon/2 laser for 488 nm wavelengths and a HeNe633 laser for 568–647 nm wavelengths. The LSM710 System is controlled via a HP Z820 workstation with Windows 7 (64 bit) operating system and ZEN 2011 software is used to control the microscope, scanning, laser module, tools, and image acquisition and processing. Twenty one images at 1Om through the z-axis were taken throughout the dentate gyrus molecular layer and representative images were selected from the z-stack.
Identification of PR-ir Cells by Ultrastructural Morphology
Procedures for PR immunocytochemistry with electron microscopy were similar to those used by Waters et al., (2008).
Tissue Preparation
On P7 (N=2) and P14 (N=2) rats were deeply anesthetized with sodium pentobarbital (150mg/kg, i.p.) and intracardially perfused with 2–5ml 0.9% saline followed by 20–50ml 3.75% acrolein and 2% paraformaldehyde in 0.1M phosphate buffer (PB, pH 7.4). Brains were removed from the skull and postfixed in fixative for 30min, then 200ml 2% paraformaldehyde in 0.1M PB. Brains were cut coronally at 40Om on a vibrating microtome and stored in cryoprotectant as described above until immunocytochemical processing.
Pre-embedding immunocytochemistry
Briefly, sections were rinsed in TBS, and then incubated in TBS containing 0.5% bovine serum albumin (BSA) for 30 min. PR antiserum (DAKO Corp.) was diluted 1:500 in TBS containing 0.1% BSA for 24 hours at room temperature (25 °C) followed by 96 hours at 4°C. Sections were rinsed in TBS and incubated for 30 min in goat anti-rabbit biotinylated IgG (1:400). Following TBS rinses, sections were incubated in ABC reagent (Vectastain Elite Kit, Vector Laboratories) for 30 min, and then rinsed again in TBS, followed by incubation in TBS containing 0.05% 3,3’ diaminobenzidine and 0.01% H2O2 for 6 min.
Electron Microscopy
Sections from the dorsal hippocampus were postfixed in 2% osmium tetroxide in PB, dehydrated, and embedded in EMBed 812 (EMS) between two sheets of Aclar plastic (Milner, Waters, Robinson, & Pierce, 2011). Ultrathin sections (70 nm) from the Epon tissue-plastic interface of each section were cut on a Leica UTC ultramicrotome, collected on 400 mesh copper grids (EMS). A Tecnai Biotwin electron microscope (FEI) equipped with an Advanced Microscopy Techniques digital camera (software version 3.2; Danvers, MA) was used to view sections. Profiles were classified as using morphological criteria described by Peters, Palay, & Webster, 1991. For figures, digital images were adjusted for levels of brightness, contrast, and sharpness (using the unsharp mask function) in Adobe PhotoShop 7.0.
Identification of PR-ir Cell Phenotype
Cell-type markers
In order to characterize the phenotype of PR immunoreactivity (PR-ir) in cells in the postnatal dentate gyrus, PR-ir was colocalized with immunoreactivity for each of the following cell-type markers (See Table 1 for primary antibodies): nestin (marker for neural precursor cells); NeuN (marker for mature neurons); MAP2 (neuronal marker); vimentin (marker for radial glial cells); calretinin (marker for Cajal-Retzius neurons); reelin (marker for Cajal-Retzius neurons).
Tissue preparation
On P7, pups were anesthetized with a lethal dose of sodium pentobarbital (i.p.), intracardially perfused, and tissue prepared as described above for GAD67.
Double Label Fluorescent Immunocytochemistry
For fluorescent double labeling, immunocytochemistry was performed as described above with the following modifications. Sections were incubated in one of the following primary antibodies (refer to Table 1): mouse anti-NeuN (1:1000); mouse anti-vimentin (1:400); mouse anti-nestin (1:400); goat anti-calretinin (1:1000); mouse anti-reelin (1:2000); mouse anti-MAP2 (1:1000) in TBS containing Triton X-100, and 2% NGS or 2% NHS for 48 hours at 4°C. After primary incubation, tissue was incubated in fluorescently tagged secondary antibody: for NeuN, vimentin, nestin and reelin: AlexaFluor 568 (1:200, goat anti-mouse, Invitrogen Inc.) in TTG buffer; for MAP-2 : AlexaFluor 488 (1:200 goat anti-mouse, Invitrogen Inc.) in TTG buffer; for calretinin: AlexaFluor 568 (1:200, donkey anti-goat, Invitrogen Inc.) in TTH buffer for 90 min at room temperature. Tissue was then rinsed for 1 hour in TBS before primary incubation in antisera against PR (1:1000, rabbit polyclonal, DAKO Corp.) in TTG/TTH buffer for 72 hours at 4°C as described above. For adequate fluorescence visualization of PR, the immunohistochemical signal was enhanced using the Tyramide Signal Amplification as described above for GAD67. After PR primary incubation, the tissue was incubated in biotinylated secondary antibody (5Og/ml, goat anti-rabbit, Vector Labs) in TTG/TTH buffer for 90 min at room temperature, followed by incubation in streptavidin-horseradish peroxidase (SA-HRP, 1:100) in TBS containing 0.3% Triton-X (TTX) for 60 min, and then incubation in Biotinyl Tyramide for 10 min in TTX buffer. Finally, tissue was incubated in a solution containing streptavidin conjugated fluorophore to detect PR (1:1000, Cy3-Streptavidin, Invitrogen Inc. for MAP-2; AlexaFluor 488, Invitrogen Inc. for all other proteins) in TBS buffer for 60 min at room temperature. Sections were then mounted on slides and coverslipped with ProLong Gold Antifade reagent (Invitrogen Inc.). Confocal microscopy was performed as described above for GAD67.
Analysis of PR-ir and Reelin-ir Colocalization
Two sections through the dorsal hippocampus (Plates 169 and 170 from Paxinos, Ashwell, & Tork, 1994) from each of four P7 males representing three litters were used for PR-ir and reelin-ir cell counts. Cells that were immunopositive for PR only, reelin only, or both PR and reelin were counted within the entire extent of both the supra- and infra-pyramidal blades of the MOL using the confocal microscope.
Behavioral Testing
Animals and RU486 treatment
Pregnant rats were allowed to deliver normally and the day of birth was designated postnatal day 1 (P1). Male rat pups received either a PR antagonist, RU486 (20mg/kg in sesame oil, s.c.) (N=13) or an equal volume of the vehicle alone (N=11) from P1-P14 as previously reported in (Lonstein, Quadros, & Wagner, 2001; Willing & Wagner, 2016).
Behavioral Testing
Prior to behavior testing, all animals were handled by the experimenter for 3 min per day for two weeks. Behavior testing commenced at P60 in the following order: Open Field, Novel Object Recognition, Novel Object Placement. Behavior was video recorded and subsequently scored by two independent observers blind to treatment group. Procedures for all three tasks were based on previously described methods (Bisagno et al., 2004; Bowman, Micik, Gautreaux, Fernandez, & Luine, 2009; Eiland & McEwen, 2012; Ennaceur & Aggleton, 1997; Ennaceur, Neave, & Aggleton, 1997; Luine, Jacome, & MacLusky, 2003; Spencer, Waters, Milner, & McEwen, 2008) The test arena used for all three behavioral tests was a Plexiglas box (70cm X 70cm X 40cm) and was cleaned with 50% ethanol between each trial.
Open Field Behavior
The Open Field test was used as a measure of general motor activity and anxiety. A grid (16 square boxes of 17.5cm each side) was placed on the floor of the arena. The inner 4 squares were designated as the “center” of the arena. The animals were acclimated to the apparatus by exploring it freely for 5 minutes. The total number of grid lines crossed (Total Grid Entries), the total number of entries into the center squares (# Central Entries), and total duration of time spent in the “center” of the arena were recorded. Significant differences between groups were analyzed with Student t tests (p<0.05).
Novel Object Recognition
The object recognition task measures long-term recognition memory and is based on the tendency of rats to explore objects that are unfamiliar. The objects used for this study were water bottles and soda cans (heavy enough that animals could not move the objects during the test, but of equal weight and dimension). The test consisted of a habituation trial (T1) and a test trial (T2), with a two hour inter trial interval (ITI). In T1, two identical objects were placed in opposite corners of the arena and the animals were placed into the arena equidistant from the two objects and allowed to explore freely for 5 minutes. Following the 2 hour ITI, one of the objects was replaced by a different novel object of similar dimension and weight. The animal was again placed into the arena equidistant from the two objects and allowed to explore freely for 5 minutes. Interaction with an object was operationally defined as touching, sniffing, whisking or looking at the object from no more than 2cm away. Both the objects themselves and the left/right position of the novel object were counterbalanced. Behavior was video recorded and analyzed by two experimenters blind to treatment group. Total interaction time with objects was measured in seconds. The percent time spent with the novel object was calculated as time (sec) spent with novel object divided by total time spent with all objects X 100. More time spent with the novel object is taken as an indication of an intact memory of the familiar object. Significant differences between groups were analyzed with Student t tests (p<0.05).
Novel Object Placement
The object placement task is a measure of long-term spatial memory. T1 of this task is identical to that for the Object Recognition test except that several spatial cues were placed throughout the testing room. After a 4 hour ITI, one of the objects is moved to a novel location within the arena. The animal is placed into the arena equidistant from both objects and allowed to explore freely for 5 minutes. Interaction with the objects was recorded and scored as described above. Significant differences between groups were analyzed with Student’s t tests (p<0.05).
RESULTS
Ontogeny of PR-ir Cell Number in dentate gyrus
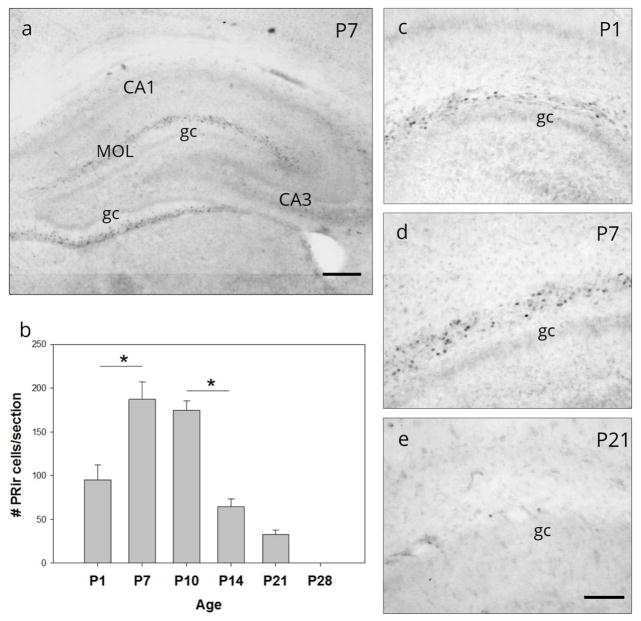
Cells with PR-ir were observed within the MOL of the supra-pyramidal and infra-pyramidal blades of the dentate gyrus throughout ventral and dorsal hippocampus from at least the day of birth (P1) through P21. PR-ir was no longer detectable at P28 (Figure 1). One-way ANOVA of PR-ir corrected cell number in the ventral dentate gyrus revealed a significant main effect of age (F(4,32) =22.73, p<0.001; Figure 1b). Post hoc analysis revealed a significant increase in PR-ir cell number between P1 and P7 (p<0.001) and a significant decrease between P10 and P14 (p<0.001). PR-ir cells were absent at P28. Nuclear PR-ir was not detected in the granule cell layer, nor in any of the CA regions.
Figure 1.
Ontogeny of progesterone receptor (PR) expression in dentate gyrus. (a) Nuclear progesterone receptor immunoreactivity (PR-ir) was observed within the molecular layer (MOL) of both the infra- and supra-pyramidal blades of the dentate gyrus (DG) of the neonatal rat. Nuclear PR-ir was not detectable in the granule cell layer (gc), nor in other regions of the hippocampus (e.g., CA1-3). (b) The number of PR-ir cells in the supra-pyramidal MOL on postnatal days 1 (P1) through P28. PR-ir cells (c) were first visible on the day of birth (P1), (d) peaked on postnatal day 7, (e) were significantly diminished by P21 and were undetectable by P28. * p<0.001; (a) Scale bar: 300Om; (c,d,e,) Scale bar: 100Om.
PR-ir is not colocalized with GAD67
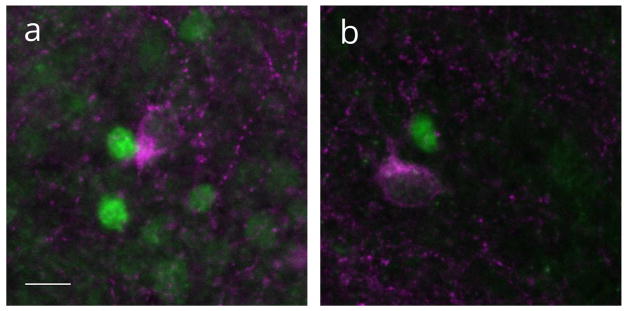
To characterize the cellular phenotype of PR-ir expressing cells within the MOL, the co-expression of PR-ir with GAD67-ir was assessed, as GABAergic interneurons comprise a significant population of cells in the MOL. Indeed, GAD67-irwas expressed in cells within the MOL of the dentate gyrus at P7 but was not colocalized with PR-ir cells (Figure 2). GAD67-ir cells were clearly distinct from PR-ir nuclei. These findings suggest that PR-ir cells were not differentiated GABAergic interneurons.
Figure 2.
Progesterone receptor (PR) expressing cells and GAD67 expressing cells are not colocalized. Nuclear PR immunoreactivity (green) and cytoplasmic GAD67 immunoreactivity (magneta) in the molecular layer of the dentate gyrus on postnatal day 7. Scale bar: 50Om.
Ultrastructural characteristics of PR-ir expressing cells in MOL
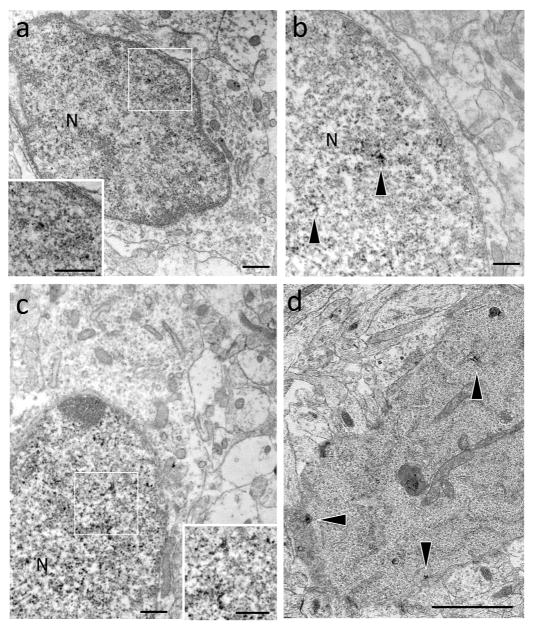
Electron microscopy was conducted to determine if cells with PR immunoreactive nucleiin MOL had the ultrastructural morphology consistent with neuronal cells or glial cells. Nuclear PR-ir was present in ovoid shaped cells with soma that were contiguous with dendrites. The neurons with nuclear PR-ir tended to have a large nucleus relative to cytoplasm with an atypically small cytoplasmic space between the nuclear membrane and the plasma membrane (Figure 3a,b). In addition, the PR-ir nuclei were located toward one end of the soma (not shown). Nuclear PR-ir was also observed in cells with morphologies consistent with degenerating neurons (i.e., smaller nuclei, dark condensed cytoplasm) (Figure 3c). In addition to the nuclear labeling, PR-ir was detected in axon terminals and dendritic spines and shafts at the ultrastructural level (data not shown) consistent with previous reports in adult hippocampus (Waters et al., 2008).
Figure 3.
At the ultrastructural level, PR-ir is contained in cells with neuronal phenotype. (a,b,c) In the molecular layer of the dentate gyrus on postnatal day 14, dense PR-ir (insets, arrows) is found in nuclei (N) of cells with neuronal morphology including an ovoid shape and dendritic process. The morphology of these neurons is atypical with relatively large nuclei compared to the cytoplasm (d) A degenerating cell, indicated by small nucleus and dark, condensed cytoplasm, with dendritic branches (not shown) contains PR-ir (arrowhead) throughout the cytoplasm. Scale bar: 500 nm
PR-ir cells in MOL do not co-express markers for neural precursor cells, radial glia nor mature neurons
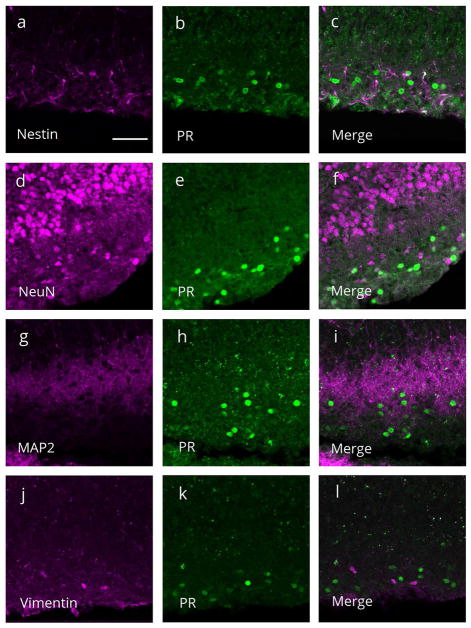
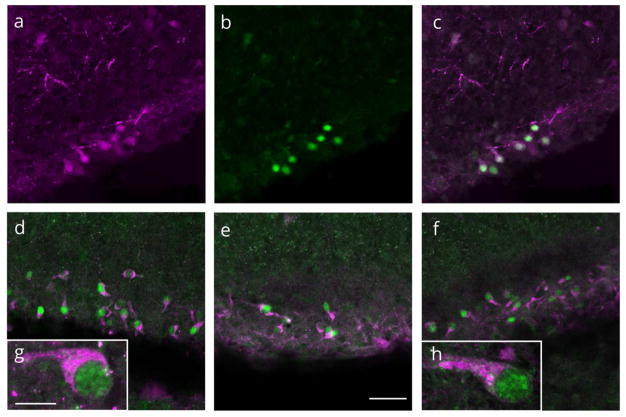
Double-label immunocytochemistry and confocal microscopy were used to determine the specific phenotype of PR immunoreactive cells in the MOL. There was no colocalization of PR-ir with nestin-ir, (marker for neural precursor cells; Figure 4A, B, C), nor with NeuN-ir (marker for mature neurons; Figure 4d,e,f) or MAP2-ir (neuronal marker; Figure 4g,h,i). Additionally, PR-ir was not colocalized with vimentin-ir (marker for radial glial cells; Figure 4j,k,l).
Figure 4.
Representative confocal images of single and merged channels through the molecular layer of the dentate gyrus on postnatal day 7 stained for progesterone receptor (PR) immunoreactivity (green) and (a–c) nestin-ir (magenta), a marker for neural precursor cells, (d–f) NeuN-ir (magenta), a marker for mature neurons, (g-i) MAP2-ir (magenta), a neuronal marker, or (j–l) vimentin-ir (magenta), a radial glial cell marker. (c,f,i,l) PR-ir was not colocalized with any of the markers. Scale bar: 50Om.
PR-ir is expressed in Cajal-Retzius cells
Dual label immunocytochemistry and confocal microscopy revealed that nearly 100% of cells with PR-ir co-express markers for Cajal-Retzius neurons. Virtually all of PR-ir cells in the MOL co-expressed the calcium binding protein, calretinin (Figure 5a,b,c). In addition, 98.2% of PR-ir cells in the MOL co-expressed the glycoprotein, reelin (Figure 5d–h). Of 553 MOL PR-ir cells counted in four animals, only 10 cells did not contain reelin-ir. Of 735 MOL reelin-ir cells counted, 182 cells did not contain PR-ir. Colocalization of PR-ir and reelin-ir was seen in both the infrapyramdial and suprapyramidal MOL layers, with greater abundance of PR/reelin expressing cells appearing in the infrapyramidal limb and particularly in the outer third of the MOL, apposed to the hippocampal fissure (Figure 1). While virtually all PR-ir cells expressed reelin, a small number of reelin expressing cells did not contain PR-ir.
Figure 5.
Virtually all progesterone receptor (PR) cells expressed caltetinin and reelin. Representative confocal images of single and merged channels through the molecular layer of the dentate gyrus stained for (a) calretinin (magenta) (b) PR (green) and (c) colocalization of PR-ir and calretinin-ir. (d–f) colocalization of PR-ir (green) and reelin-ir (magenta). (g.h) PR/reelin expressing neurons had a ‘tadpole’ morphology typical of Cajal-Retzius neurons. (a-f) Scale bar: 50Om. (g,h) Scale bar: 10Om.
PR-ir/reelin immunoreactive cells had a distinct morphology, with an ovoid cell body, a large nucleus, and a thick immunoreactive process emerging from one pole of the soma (Figure 5g,h). These characteristics are consistent with the typical ‘tadpole’ morphology of Cajal-Retzius neurons.
Impairment in memory tasks by inhibition of PR activity during development
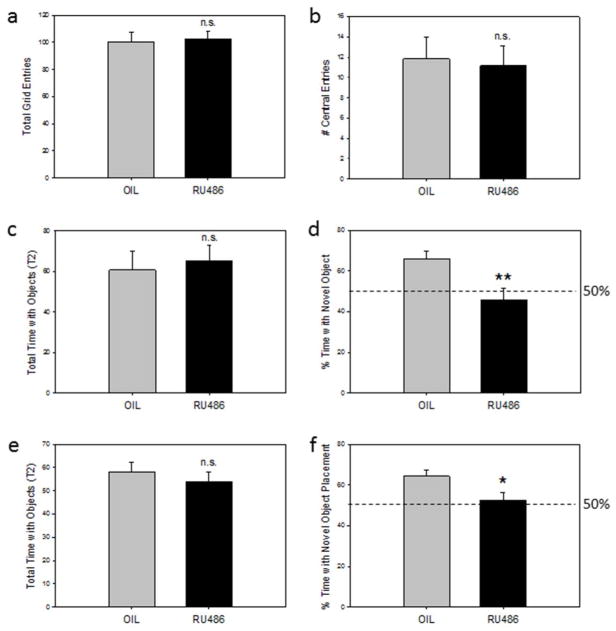
Open Field Test
There was no significant difference in the total number of grid crossings between vehicle and RU486 treated animals (Figure 6a), indicating no effect of PR inhibition on general motor activity. Similarly, there was no significant difference between the two groups in the number of entries into the central grid (Figure 6b), nor in the total time spent in the center grid (Oil: 7.43 ± 1.25; RU486: 6.55 ±1.32) indicating no effect of RU486 on generalized anxiety.
Figure 6.
(a,b) Open Field Test: (a) The total number of grid entries and (b ) the total number of entries into the center square in adult rats treated with the PR antagonist, RU486, or the oil vehicle from postnatal day 1 (P1) through P14. (n.s. not significant). (c,d) Novel Object Recognition Task: (c) The total amount of time (sec) spent with objects in test trial 2 and (d) the percent of time spent with the novel object in RU486 and oil treated rats. (50% time equals chance) (n.s. not significant; **p<0.05). (e,f) Novel Object Placement Task: (e) The total time (sec) spent with objects in test trial 2 and (f) the percent of time spent with the object moved to a novel location in RU486 and oil treated rats. (50% time equals chance) (n.s. not significant; *p<0.05).
Object Recognition Test
There were no significant differences between vehicle and RU486 treated animals in the total time interacting with objects in either Trial 1 (Oil 66.84 ±7.10; RU486: 49.04 ±6.19) nor in Trial 2 (Figure 6c), suggesting that the groups explored objects equally. In contrast, the percent of time spent interacting with the novel object was significantly reduced in RU486 treated animals compared with oil treated animals in Trial 2 (p< 0.05) (Figure 6d). Ratios of the time spent with the novel object to the total time spent with all objects were as follows: mean time novel: mean time total = Oil group: 38.24s (5.58): 60.81s (9.14); RU486 group: 31.98s (5.81): 65.42s (7.49).
Object Placement Test
There were no significant differences between oil treated and RU486 treated animals in the total time interacting with objects in either Trial 1 (Oil: 44.94 ±7.34; RU486: 36.05 ±4.52) nor in Trial 2 (Figure 6e). However, the percent of time spent interacting with the object in a new location was reduced in RU486 treated animals compared to controls in Trial 2 (p<0.05) (Figure 6f). Ratios of the time spent with the novel placed object to the total time spent with all objects were as follows: mean time (sec) novel: mean time (sec) total = Oil group: 36.54 (2.53): 58.08 (4.25); RU486 group: 27.96 (3.07): 53.72 (4.52).
DISCUSSION
The present study identifies a novel population of PR expressing cells in the dentate gyrus of the postnatal rat. PR was transiently expressed in cells of the MOL of the dentate gyrus during the first three weeks of life. Although PR-ir cells did not co-express typical markers for mature neurons, these cells demonstrated a neuronal ultrastructural phenotype, but with an atypical somal morphology. Further, neurochemical identification of these cells indicated that PR expressing cells are Cajal-Retzius neurons, a type of “pioneer” neuron localized to the MOL, critical for the proper development of the dentate gyrus and its integration into the hippocampal circuitry. Inhibition of PR activity during postnatal life impaired subsequent performance on hippocampus-dependent/related tasks in adulthood. Together, these results suggest that PR activity in the MOL may play an important role in establishing proper dentate gyrus circuitry, thereby ensuring normal recognition memory function later in life.
Ontogeny of PR-ir cells in Dentate Gyrus
PR immunoreactive cells were localized to the MOL of both the supra-pyramidal and infra-pyramidal blades of the dentate gyrus throughout the septo-temporal extent of the hippocampus. PR-ir cells were present on the day of birth, reaching peak numbers between P7 and P10 with a significant decline in cell number by P14 and P21. By P28, PR-ir cells were no longer observed in the MOL. There were no PR-ir cells detectable within the granule cell layer of the dentate gyrus, nor within the CA regions of the hippocampus. To date, study of steroid receptor expression in the hippocampus has primarily been limited to adulthood (Loy, Gerlach, & McEwen, 1988; Milner et al., 2001; Mitra, Hoskin, Yudkovitz, & Pear, 2003; Waters et al., 2008), with a particular focus on the role of estrogen receptor in functional and structural synaptic plasticity of principal neurons in the dentate gyrus and CA regions (Mukai et al., 2007, 2010; Oberlander & Woolley, 2016; Tanaka & Sokabe, 2013). Only a very limited literature exists regarding steroid hormone receptor expression in the dentate gyrus during development (Bender et al., 2010) and to date, there have been no reports on the presence or function of PR. The transient expression of high levels of PR in a relatively large population of cells within the postnatal MOL is unique and suggests that this transcription factor may play a previously overlooked role in the early shaping of hippocampal circuitry.
PR-ir Cells are Cajal-Retzius Neurons
GABAergic interneurons are the predominant neuronal subtype in the MOL, with molecular layer perforant path associated (MOPP) cells occupying the inner MOL and subiculum-projecting neurons localized in the outer MOL (Armstrong et al., 2012; Ceranik et al., 1997; Halasy & Somogyi, 1993; Han, Buhl, & Lorincziln, 1993). However, PR-ir and GAD67-ir were not colocalized (0%), and were clearly contained in distinct cell populations within the MOL. Additionally, PR-ir cells did not co-express the radial glial cell marker, vimentin, and indeed, ultrastructural morphology indicated that PR-ir was present in the nuclei of neurons, albeit with unique features including a large nucleus relative to cytoplasm. PR-ir cells did not co-express the neuronal marker NeuN, nor did they co-express nestin, a marker for neural precursor cells. However, virtually 100% of PR-ir expressing cells co-expressed calretinin and reelin, consistent with the phenotype of Cajal-Retzius neurons, a transient population of developmentally critical, pioneer neurons in the dentate gyrus. Cajal-Retzius neurons uniquely express these proteins in the marginal zones of neocortex, and in hippocampus during early development (Del Río, Martinez, Fonseca, Auladell, & Soriano, 1995; Del Río et al., 1997; Hirotsune et al., 1995; Jiang & Swann, 1997; Ogawa et al., 1995; Sheldon et al., 1997).
Additional findings support the idea that PR is expressed in Cajal-Retzius neurons in the MOL. First, the morphology of PR expressing cells, observed at both the ultrastructural and light level, are highly consistent with Cajal-Retzius neurons, as PR-ir cells exhibited the distinct bipolar morphology (i.e., tadpole shape) with a relatively large nucleus situated toward one end of the soma, that distinguishes Cajal-Retzius neurons (Anstotz et al., 2016; von Haebler, Stabel, Draguhn, & Heinemann, 1992). Second, PR-ir cells are localized specifically to the outer third of the MOL, as are Cajal-Retzius neurons (J. a Del Río et al., 1997). Third, previous research has shown that Cajal-Retzius neurons reach their postnatal position in the developing supra and infra-pyramidal blades of the dentate gyrus around the time of birth in the rat (Del Río et al., 1995; Soriano et al., 1994), coincident with the first appearance of PR-ir in cells of this region. Fourth, Cajal-Retzius neurons represent a transient population of neurons within the dentate gyrus with numbers peaking during the first postnatal week, similar to PR-ir cell number. However, beginning in the second postnatal week, Cajal-Retzius neuron numbers begin to decline and are greatly reduced by the third postnatal week (Del Río et al., 1996; Supèr et al., 1998). This decline in Cajal-Retzius neurons is concomitant with the decrease in PR-ir cell number in the MOL, with a dramatic decrease between P10 and P14, and an absence by P28. Indeed, at the ultrastructural level, PR immunoreactivity was also observed within small, electron-dense, granulated nuclei, suggesting that PR is present in neurons undergoing an intermediate stage of neuronal degeneration. Taken together, these results strongly indicate that PR is expressed in a population of highly specialized, pioneer neurons that play a critical role in the development of hippocampal circuitry.
PR Antagonist during Postnatal Life Impairs Memory in Adulthood
Inhibition of PR activity during the first two weeks of life impaired performance on two recognition memory tasks in adulthood. In the Novel Object Recognition task and the Novel Object Placement task, rats will typically spend more time with an unfamiliar object or a familiar object moved to a new location compared to a familiar object in a familiar location. These tasks are used to test facets of episodic memory, namely recognition memory and memory for object location in a context, both of which are mediated by medial temporal lobe circuits that ultimately terminate in the dentate gyrus (for review, see Knierim, Neunuebel, & Deshmukh, 2014; Squire, Stark, & Clark, 2004; Squire, Wixted, & Clark, 2007). Animals treated postnatally with the PR antagonist, RU486, spent significantly less time with a non-familiar object and less time with a familiar object in a new location compared to controls, suggesting impairments in both aspects of episodic memory. Because RU486 is a glucocorticoid receptor (GR) antagonist, as well as a PR antagonist, behavioral effects mediated by GR activity cannot be ruled out. However, several factors suggest it is unlikely that the present behavioral findings are mediated by GR. Expression of GR protein and mRNA is low and largely restricted to granule cells in the dentate gyrus during the first 10 postnatal days (Galeeva, Ordyan, Pivina, & Pelto-Huikko, 2006; Lawson, Ahima, Krozowski, & Harlan, 1991; van Eekelen, Bohn, & de Kloet, 1991; Yi, Masters, & Baram, 1994). Additionally, the first two postnatal weeks in the rat are typified by extremely low levels of circulating corticosterone, the ligand for GR (Meaney, Sapolsky, & McEwen, 1985; Rosenfeld, van Eekelen, Levine, & de Kloet, 1993; Sapolsky & Meaney, 1986). As such, stressors do not elicit corticosterone release during this developmental period (Cote & Yasumura, 1975). Importantly, present results demonstrate that rats treated with RU486 during the first two weeks of life did not differ in the number of central grid entries or time spent in the center grid in an open field test, suggesting that adult anxiety-like behavior was not altered by the antagonist.
These findings are consistent with the hypothesis that PR activity may be important for the proper development of afferent connections to the MOL. Lateral perforant path afferents from the perirhinal cortex and the lateral entorhinal cortex project to the outer third of the MOL (Amaral et al., 2007; Hayashi & Nonaka, 2011; M.P. Witter et al., 2000; Menno P. Witter, 2007) and initially make synaptic connections with Cajal-Retzius neurons, eventually forming permanent synapses with granule cell dendrites later in development. In general, this circuit, mediates both object recognition and object-in-place memory (Deshmukh, Johnson, & Knierim, 2013; Deshmukh & Knierim, 2011; Keene et al., 2016; Kuruvilla & Ainge, 2017; Van Cauter et al., 2013). More specifically, cytotoxic lesions of the perirhinal cortex impaired performance on the Novel Object Recognition task (Barker & Warburton, 2011; Mumby & Pinel, 1994) whereas lesions of the lateral entorhinal cortex impaired the ability to associate an object with a place or a context (Kuruvilla & Ainge, 2017; Wilson et al., 2013). Pharmacological inactivation of the lateral perforant path itself impaired both the ability to discern familiar objects from novel objects, and to associate those objects with a specific place (Hunsaker, Mooy, Swift, & Kesner, 2007). Taken together, these findings inform the behavioral results of the present study. Since inhibiting PR activity during development was sufficient to impair both object recognition and object place association, and disabling the lateral perforant path in adulthood also disrupts both behaviors, it can be hypothesized that PR activity in Cajal-Retzius neurons within the MOL may ensure proper innervation of the MOL by the lateral perforant path. Consistent with this hypothesis, PR-ir Cajal-Retzius cells are located in the outer MOL of the dentate gyrus, the synaptic target region of lateral perforant path afferents from the lateral entorhinal cortex and the perirhinal cortex (Amaral et al., 2007; Hayashi & Nonaka, 2011).
Potential Role of PR Expression in Cajal-Retzius Neuron Function
PR is expressed in Cajal-Retzius neurons during the postnatal period, which is a critical phase of development for the dentate gyrus and its primary afferent input in the rat (Bayer, 1980a, 1980b; V Borrell, Ruiz, Del Río, & Soriano, 1999; Deng et al., 2007; Navarro-quiroga, Hernandez-valdes, & Lin, 2006; Schlessinger, Cowan, & Gottlieb, 1975; Supèr & Soriano, 1994). Additionally, behavioral data from the present study suggest that PR activity during this period is necessary for functional connectivity of the dentate gyrus. As a nuclear transcription factor, PR could potentially regulate fundamental cellular processes within Cajal-Retzius neurons and thereby impact the critical role Cajal-Retzius neurons play in shaping the nascent dentate gyrus and its circuitry. Cajal-Retzius neurons serve two major roles in development of the dentate gyrus; ensuring proper granule cell layer anatomy and establishing proper connections onto granule cell dendrites within the MOL, the former through the secretion of the glycoprotein reelin and the latter through the formation of temporary synapses with arriving entorhinal afferents. PR activity could influence either or both of these processes.
Cajal-Retzius neurons express and secrete the extracellular matrix protein reelin (D’Arcangelo et al., 1995; Hirotsune et al., 1995; Ogawa et al., 1995) which is responsible for orchestrating fundamental developmental mechanisms such as neuronal migration, axon target finding, and dendrite maturation in the dentate gyrus (i.e. J. a Del Río et al., 1997; Frotscher, Zhao, & Förster, 2007; Niu, Yabut, & D’Arcangelo, 2008). One possibility is that PR, as a powerful transcription factor, may influence hippocampal development through the regulation of reelin expression. Mutation of the reelin protein in mice (i.e., producing reeler mice) resulted in profound changes in neuronal migration with an absence of a distinct laminated granule cell layer, and granule cells scattered ectopically throughout the dentate gyrus (Frotscher et al., 2007; Zhao et al., 2004). This appears to be the result of failure of the radial glial scaffold to align radially (Frotscher, Haas, & Forster, 2003; Weiss et al., 2003).
While we did not observe gross differences in granule cell layer morphology (e.g., ectopic cell localization) of rats treated postnatally with RU486 (unpubl. obs.), this does not preclude more subtle, but critical, alterations in granule cell layer architecture. For example, in heterozygous Reeler mice, in which reelin is reduced but not absent, there is little disruption in the gross morphology of the granule cell layer. However, dissociated hippocampal neurons of heterozygous Reeler mice show reduced numbers of dendritic spines, shorter spines, and reductions in molecular markers of synaptic machinery such as PSD-95 and NR2A subunits, reflecting lower levels of synaptic maintenance (S. Niu et al., 2008; Sanyong Niu, Renfro, Quattrocchi, Sheldon, & D’Arcangelo, 2004). Conversely, overexpression of reelin produces larger more complex spines, longer synaptic contacts and enriched spine apparatus in the outer MOL (Bosch, Muhaisen, Pujadas, Soriano, & Martínez, 2016; Pujadas et al., 2010). Taken together, these findings support the idea that PR regulation of reelin expression could be one mechanism by which the granule cell layer undergoes proper development, thereby ensuring typical recognition memory later in life.
Cajal-Retzius neurons also function as pioneer neurons during the development of the perforant path, the main cortical input to the hippocampus, a process that is largely independent of reelin (i.e., the perforant path forms normally in Reeler mice) (Victor Borrell et al., 2007; J. a Del Río et al., 1997; Wu, Li, Yu, & Deng, 2008; Zhao, Förster, Chai, & Frotscher, 2003). Terminals from the entorhinal cortex make temporary functional synapses with Cajal-Retzius neurons in the MOL, before ultimately forming mature synapses with dendrites of granule cells (Supèr et al., 1998). Cajal-Retzius neurons likely guide pathway formation as they extend across the hippocampal fissure into the subiculum and entorhinal cortex (Ceranik et al., 1999; Ceranik, Zhao, & Frotscher, 2000) and may serve as a pathway for entorhinal axons entering into the MOL. Indeed, excitotoxic lesions of Cajal-Retzius neurons in hippocampal/entorhinal cocultures prevented entorhinal afferents from establishing their layer-specific synaptic targets within the MOL (Del Río et al., 1997). Cajal-Retzius neurons can also induce axon regeneration from adult entorhinal cortex in hippocampal cocultures, eliciting axon growth from developmentally quiescent cortex, illustrating Cajal-Retzius neurons’ powerful chemoattractive effect (Del Río, Solé, Borrell, Martínez, & Soriano, 2002). Changes in PR transcriptional activity during development could impact the target phenotype of Cajal-Retzius neurons, thereby altering axon pathfinding of perforant path afferents and/or influencing mechanisms of synaptogenesis between entorhinal axons and granule cell dendrites in the MOL. This would profoundly alter Cajal-Retzius pioneer neuron function and alter hippocampal circuitry and subsequent behavior.
Conclusions
The present study demonstrates that PR, a powerful transcription factor, is expressed in Cajal-Retzius neurons of the molecular layer during a critical period of development for the perforant path and for dentate gyrus structure and circuitry. Inhibition of PR activity during this period impaired both recognition and contextual facets of episodic memory in adulthood, both of which are associated with hippocampal and entorhinal cortex afferent function. The present findings are consistent with the idea that PR activity during development ensures the proper target phenotype and function of a population of developmentally-critical pioneer cells. Perturbations in progesterone levels or exposure to exogenous progestins during this period could potentially interfere with the development of normal hippocampal circuitry and disrupt subsequent learning and memory later in life.
Acknowledgments
Grant Support: NIH HD07643001 (CKW), NIH DA08259 (TAM), NS07080 (BSM);
References
- Altman J, Bayer S. Atlas of Prenatal Rat Brain Development. Boca Raton: CRC Press Inc; 1995. [Google Scholar]
- Amaral DG, Scharfman HE, Lavenex P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies) Progress in Brain Research. 2007:163. doi: 10.1016/S0079-6123(07)63001-5. [DOI] [PMC free article] [PubMed]
- Anstotz M, Huang H, Marchionni I, Haumann I, Maccaferri G, Lu bke JHR. Developmental Profile, Morphology, and Synaptic Connectivity of Cajal-Retzius Cells in the Postnatal Mouse Hippocampus. Cerebral Cortex. 2016 Feb;:1–18. doi: 10.1093/cercor/bhv271. [DOI] [PMC free article] [PubMed]
- Armstrong C, Szabadics J, Tamas G, Soltesz I. Neurogliaform Cells in the Molecular Layer of the Dentate Gyrus as Feed-Forward γ-Aminobutyric Acidergic Modulators of Entorhinal-Hippocampal Interplay. Journal of Comparative Neurology. 2012;519(8):1476–1491. doi: 10.1016/j.biotechadv.2011.08.021.Secreted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GRI, Warburton EC. When Is the Hippocampus Involved in Recognition Memory? Journal of Neuroscience. 2011;31(29):10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA. Development of the Hippocampal Region in the Rat I. Neurogenesis Examin with H3-Thymidine Autoradiography. Journal of Comparative Neurology. 1980a;190:87–114. doi: 10.1002/cne.901900107. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Development of the Hippocampal Region in the Rat II. Morphogenesis During Embryonic and Early Postnatal Life. Journal of Comparative Neurology. 1980b;190:115–134. doi: 10.1002/cne.901900108. [DOI] [PubMed] [Google Scholar]
- Bender RA, Zhou L, Wilkars W, Fester L, Lanowski JS, Paysen D, … Rune GM. Roles of 17β-estradiol involve regulation of reelin expression and synaptogenesis in the dentate gyrus. Cerebral Cortex. 2010;20(12):2985–2995. doi: 10.1093/cercor/bhq047. [DOI] [PubMed] [Google Scholar]
- Binder LI, Frankfurter A, Kim H, Caceres A, Payne MR, Rebhun LI. Heterogeneity of microtubule-associated protein 2 during rat brain development. Proceedings of the National Academy of Sciences of the United States of America. 1984;81(17):5613–7. doi: 10.1073/pnas.81.17.5613. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6591209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisagno V, Grillo CA, Piroli GG, Giraldo P, McEwen B, Luine VN. Chronic stress alters amphetamine effects on behavior and synaptophysin levels in female rats. Pharmacology, Biochemistry, and Behavior. 2004;78(3):541–50. doi: 10.1016/j.pbb.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Borrell V, Pujadas L, Simo S, Dura D, Sole M, Cooper JA, … Soriano E. Reelin and mDab1 regulate the development of hippocampal connections. Molecular and Cellular Neuroscience. 2007;36(2):158–173. doi: 10.1016/j.mcn.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Borrell V, Ruiz M, Del Río Ja, Soriano E. Development of commissural connections in the hippocampus of reeler mice: evidence of an inhibitory influence of Cajal-Retzius cells. Experimental Neurology. 1999;156(2):268–282. doi: 10.1006/exnr.1999.7022. http://doi.org/S001448869997022X [pii] [DOI] [PubMed] [Google Scholar]
- Bosch C, Muhaisen A, Pujadas L, Soriano E, Martínez A. Reelin Exerts Structural, Biochemical and Transcriptional Regulation Over Presynaptic and Postsynaptic Elements in the Adult Hippocampus. Frontiers in Cellular Neuroscience. 2016 May;10:1–15. doi: 10.3389/fncel.2016.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman RE, Micik R, Gautreaux C, Fernandez L, Luine VN. Sex-dependent changes in anxiety, memory, and monoamines following one week of stress. Physiology & Behavior. 2009;97(1):21–29. doi: 10.1016/j.physbeh.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Caceres A, Binder LI, Payne MR, Bender P, Rebhun L, Steward O. Differential subcellular localization of tubulin and the microtubule-associated protein MAP2 in brain tissue as revealed by immunocytochemistry with monoclonal hybridoma antibodies. The Journal of Neuroscience>: The Official Journal of the Society for Neuroscience. 1984;4(2):394–410. doi: 10.1523/JNEUROSCI.04-02-00394.1984. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6699682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone DL, Handa RJ. Sex and stress hormone influences on the expression and activity of brain-derived neurotrophic factor. Neuroscience. 2013;239:295–303. doi: 10.1016/j.neuroscience.2012.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceranik K, Bender R, Geiger JR, Monyer H, Jonas P, Frotscher M, Lübke J. A novel type of GABAergic interneuron connecting the input and the output regions of the hippocampus. The Journal of Neuroscience>: The Official Journal of the Society for Neuroscience. 1997;17(14):5380–5394. doi: 10.1523/JNEUROSCI.17-14-05380.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceranik K, Deng J, Heimrich B, Lubke J, Zhao S, Forster E, Frotscher M. Hippocampal Cajal-Retzius cells project to the entorhinal cortex: Retrograde tracing and intracellular labelling studies. European Journal of Neuroscience. 1999;11(12):4278–4290. doi: 10.1046/j.1460-9568.1999.00860.x. [DOI] [PubMed] [Google Scholar]
- Ceranik K, Zhao S, Frotscher M. Development of the entorhino-hippocampal projection: guidance by Cajal-Retzius cell axons. Annals of the New York Academy of Sciences. 2000;911:43–54. doi: 10.1111/j.1749-6632.2000.tb06718.x. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. Journal of Neurobiology. 2005;64(1):34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- Cote TE, Yasumura S. Effect of ACTH and Histamine Stress on Serum Corticosterone and Adrenal Cyclic AMP. Endocrinology. 1975;96(4):1044–1047. doi: 10.1210/endo-96-4-1044. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6182130. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1007/s00330-011-2364-3. [DOI] [PubMed] [Google Scholar]
- de Bergeyck V, Naerhuyzen B, Goffinet AM, Lambert de Rouvroit C. A panel of monoclonal antibodies against reelin, the extracellular matrix protein defective in reeler mutant mice. Journal of Neuroscience Methods. 1998;82(1):17–24. doi: 10.1016/s0165-0270(98)00024-7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10223511. [DOI] [PubMed] [Google Scholar]
- Del Rio JA, Martinez A, Fonseca M, Auladell C, Soriano E. Glutamate-like Immunoreactivity and Fate of Cajal-Retzius Cells in the Murine Cortex as Identified with Calretinin Antibody. Cerebral Cortex. 1995;(1):13–21. doi: 10.1093/cercor/5.1.13. [DOI] [PubMed] [Google Scholar]
- Del Río JA, Martinez A, Fonseca M, Auladell C, Soriano E. Glutamate-like immunoreactivity and fate of Cajal-Retzius cells in the murine cortex as identified with calretinin antibody. Cerebral Cortex. 1995;5(1):13–21. doi: 10.1680/udap.2010.163. [DOI] [PubMed] [Google Scholar]
- Del Río JA, Solé M, Borrell V, Martínez A, Soriano E. Involvement of Cajal-Retzius cells in robust and layer-specific regeneration of the entorhino-hippocampal pathways. European Journal of Neuroscience. 2002;15(12):1881–1890. doi: 10.1046/j.1460-9568.2002.02027.x. [DOI] [PubMed] [Google Scholar]
- Del Río Ja, Heimrich B, Borrell V, Förster E, Drakew A, Alcántara S, … Soriano E. A role for Cajal-Retzius cells and reelin in the development of hippocampal connections. Nature. 1997;385:70–74. doi: 10.1038/385070a0. [DOI] [PubMed] [Google Scholar]
- Del Río Ja, Heimrich B, Supèr H, Borrell V, Frotscher M, Soriano E. Differential survival of Cajal-Retzius cells in organotypic cultures of hippocampus and neocortex. The Journal of Neuroscience>: The Official Journal of the Society for Neuroscience. 1996;16(21):6896–6907. doi: 10.1523/JNEUROSCI.16-21-06896.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng JB, Yu DM, Wu P, Li MS. The tracing study of developing entorhino-hippocampal pathway. International Journal of Developmental Neuroscience. 2007;25(4):251–258. doi: 10.1016/j.ijdevneu.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Deshmukh SS, Johnson JL, Knierim JJ. Perirhinal cortex represents nonspatial, but not spatial, information in rats foraging in the presence of objects: Comparison with lateral entorhinal cortex. Hippocampus. 2013;22(10) doi: 10.1002/hipo.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perirhinal Deshmukh SS, Knierim JJ. Representation of non-spatial and spatial information in the lateral entorhinal cortex. Frontiers in Behavioral Neuroscience. 2011 Oct;5:1–33. doi: 10.3389/fnbeh.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron. 2014;83(4):764–770. doi: 10.1007/s11103-011-9767-z.Plastid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L, McEwen BS. Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus. 2012;22(1):82–91. doi: 10.1002/hipo.20862. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Aggleton JP. The effects of neurotoxic lesions of the perirhinal cortex combined to fornix transection on object recognition memory in the rat. Behavioural Brain Research. 1997;88(2):181–93. doi: 10.1016/s0166-4328(97)02297-3. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9404627. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Experimental Brain Research. 1997;113(3):509–19. doi: 10.1007/pl00005603. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9108217. [DOI] [PubMed] [Google Scholar]
- Forger NG. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138(3):929–938. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on hippocampus. Nature. 2004;431:188–191. doi: 10.1038/nmeth.2250.Digestion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M, Haas CA, Forster E. Reelin controls granule cell migration in the dentate gyrus by acting on the radial glial scaffold. Cerebral Cortex. 2003;13:634–640. doi: 10.1093/cercor/13.6.634. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Zhao S, Förster E. Development of cell and fiber layers in the dentate gyrus. Progress in Brain Research. 2007:163. doi: 10.1016/S0079-6123(07)63007-6. [DOI] [PubMed]
- Galeeva A, Ordyan N, Pivina S, Pelto-Huikko M. Expression of glucocorticoid receptors in the hippocampal region of the rat brain during postnatal development. Journal of Chemical Neuroanatomy. 2006;31(3):216–225. doi: 10.1016/j.jchemneu.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Melcangi RC. Steroids and Glial Cell Function. Glia. 2006;54:485–498. doi: 10.1002/glia. [DOI] [PubMed] [Google Scholar]
- Gulyás AI, Hájos N, Freund TF. Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. The Journal of Neuroscience>: The Official Journal of the Society for Neuroscience. 1996;16(10):3397–3411. doi: 10.1523/JNEUROSCI.16-10-03397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasy K, Somogyi P. Subdivisions in the Multiple GABAergic Innervation of Granule Cells in the Dentate Gyrus of the Rat Hippocampus. European Journal of Neuroscience. 1993;5:411–429. doi: 10.1111/j.1460-9568.1993.tb00508.x. [DOI] [PubMed] [Google Scholar]
- Han Z, Buhl EH, Lorincziln Z. A High Degree of Spatial Selectivity in the Axonal and Dendritic Domains of PhysiologicalG Identified Local-circuit Neurons in the Dentate Gyrus of the Rat Hippocampus. European Journal of Neuroscience. 1993;5:395–410. doi: 10.1111/j.1460-9568.1993.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Hartley T, Lever C, Burgess N, O’Keefe J. Space in the brain: how the hippocampal formation supports spatial cognition. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2014;369(1635):20120510. doi: 10.1098/rstb.2012.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Nonaka Y. Cooperation and competition between lateral and medial perforant path synapses in the dentate gyrus. Neural Networks. 2011;24(3):233–246. doi: 10.1016/j.neunet.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Hirotsune S, Takahara T, Sasaki N, Hirose K, Yoshiki A, Ohashi T, … Hayashizaki Y. The reeler gene encodes a protein with an EGF-like motif expressed by pioneer neurons. Nature Genetics. 1995;10:77–83. doi: 10.1038/ng0595-77. [DOI] [PubMed] [Google Scholar]
- Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. The Journal of Neuroscience>: The Official Journal of the Society for Neuroscience. 1985;5(12):3310–28. doi: 10.1523/JNEUROSCI.05-12-03310.1985. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/4078630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR. Interneurons of the dentate gyrus: an overview of cell types, terminal fields and neurochemical identity. Progress in Brain Research. 2007;163:217–232. doi: 10.1680/udap.2010.163. [DOI] [PubMed] [Google Scholar]
- Hull EM, Franz JR, Snyder AM, Nishita JK. Perinatal Progesterone and Learning, Social and Reproductive Behavior in Rats. Cell and Tissue Research. 1980;24:251–256. doi: 10.1007/s00330-011-2364-3. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Mooy GG, Swift JS, Kesner RP. Dissociations of the medial and lateral perforant path projections into dorsal DG, CA3, and CA1 for spatial and nonspatial (visual object) information processing. Behavioral Neuroscience. 2007;121(4):742–50. doi: 10.1037/0735-7044.121.4.742. [DOI] [PubMed] [Google Scholar]
- Ismail PM, Li J, DeMayo FJ, O’Malley BW, Lydon JP. A Novel LacZ Reporter Mouse Reveals Complex Regulation of the Progesterone Receptor Promoter During Mammary Gland Development. Molecular Endocrinology. 2002;16(11):2475–2489. doi: 10.1210/me.2002-0169. [DOI] [PubMed] [Google Scholar]
- Izant JG, McIntosh JR. Microtubule-associated proteins: a monoclonal antibody to MAP2 binds to differentiated neurons. Proceedings of the National Academy of Sciences of the United States of America. 1980;77(8):4741–5. doi: 10.1073/pnas.77.8.4741. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7001466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahagirdar V, Wagner CK. Ontogeny of progesterone receptor expression in the subplate of fetal and neonatal rat cortex. Cerebral Cortex (New York, N.Y.>: 1991) 2010;20(5):1046–52. doi: 10.1093/cercor/bhp165. [DOI] [PubMed] [Google Scholar]
- Jiang M, Swann JW. Expression of Calretinin in Diverse Neuronal Populations During Development of Rat Hippocampus. Neuroscience. 1997;81(4):1137–1154. doi: 10.1016/s0306-4522(97)00231-5. [DOI] [PubMed] [Google Scholar]
- Kaufman DL, Houser CR, Tobin AJ. Two Forms of the γ-Aminobutyric Acid Synthetic Enzyme Glutamate Decarboxylase Have Distinct Intraneuronal Distributions and Cofactor Interactions. Journal of Neurochemistry. 1991;56(2):720–723. doi: 10.1111/j.1471-4159.1991.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene CS, Bladon J, Mckenzie S, Liu CD, Keefe JO, Eichenbaum H. Complementary Functional Organization of Neuronal Activity Patterns in the Perirhinal , Lateral Entorhinal , and Medial Entorhinal Cortices. Journal of Neuroscience. 2016;36(13):3660–3675. doi: 10.1523/JNEUROSCI.4368-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, Neunuebel JP, Deshmukh SS. Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local-global reference frames. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2014;369(1635):20130369. doi: 10.1098/rstb.2013.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruvilla MV, Ainge JA. Lateral Entorhinal Cortex Lesions Impair Local Spatial Frameworks. Frontiers in Systems Neuroscience. 2017 May;11:1–12. doi: 10.3389/fnsys.2017.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston RF, Wood ER. Associative recognition and the hippocampus: Differential effects of hippocampal lesions on object-place, object-context and object-place-context memory. Hippocampus. 2010;20(10):1139–1153. doi: 10.1002/hipo.20714. [DOI] [PubMed] [Google Scholar]
- Lawson A, Ahima R, Krozowski Z, Harlan R. Postnatal development of corticosteroid receptor immunoreactivity in the rat hippocampus. Developmental Brain Research. 1991;62:69–79. doi: 10.1016/0165-3806(91)90191-k. [DOI] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60(4):585–95. doi: 10.1016/0092-8674(90)90662-x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1689217. [DOI] [PubMed] [Google Scholar]
- Li Y, Stam FJ, Aimone JB, Goulding M, Callaway EM, Gage FH. Molecular layer perforant path-associated cells contribute to feed-forward inhibition in the adult dentate gyrus. - Supporting Information. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(22):9106–11. doi: 10.1073/pnas.1306912110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind D, Franken S, Kappler J, Jankowski J, Schilling K. Characterization of the neuronal marker NeuN as a multiply phosphorylated antigen with discrete subcellular localization. Journal of Neuroscience Research. 2005;79(3):295–302. doi: 10.1002/jnr.20354. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Quadros PS, Wagner CK. Effects of neonatal RU486 on adult sexual, parental, and fearful behaviors in rats. Behavioral Neuroscience. 2001;115(1):58–70. doi: 10.1037/0735-7044.115.1.58. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11256453. [DOI] [PubMed] [Google Scholar]
- López V, Wagner CK. Progestin receptor is transiently expressed perinatally in neurons of the rat isocortex. The Journal of Comparative Neurology. 2009;512(1):124–39. doi: 10.1002/cne.21883. [DOI] [PubMed] [Google Scholar]
- Loy R, Gerlach JL, McEwen BS. Autoradiographic localization of estradiol-binding neurons in the rat hippocampal formation and entorhinal cortex. Developmental Brain Research. 1988;39:245–251. doi: 10.1016/0165-3806(88)90028-4. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, MacLusky NJ. Rapid Enhancement of Visual and Place Memory by Estrogens in Rats. Endocrinology. 2003;144(7):2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Fisahn A. Interneurons unbound. Nature Reviews. Neuroscience. 2001;2(1):11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Sapolsky RM, McEwen BS. The Development of the Glucocorticoid Receptor System in the Rat Limbic Brain. I Ontogeny and Autoregulation. Developmental Brain Research. 1985;18:159–164. doi: 10.1007/s00330-011-2364-3. [DOI] [PubMed] [Google Scholar]
- Milner TA, Ewen BSMC, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural Evidence That Hippocampal Alpha Estrogen Receptors Are Located at Extranuclear Sites. 2001 Aug;371:355–371. 2000. [PubMed] [Google Scholar]
- Milner TA, Waters EM, Robinson DC, Pierce JP. In: Neurodegeneration: Methods and Protocols. Manfredi G, Kawamata H, editors. Vol. 793. Springer Science+Business Media LLC; 2011. [DOI] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L. Immunolocalization of Estrogen Receptor B in the Mouse Braing: Comparison with Estrogen Receptor a. Endocrinology. 2003;144(5):2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1007/s00330-011-2364-3. [DOI] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. The Journal of Neuroscience>: The Official Journal of the Society for Neuroscience. 1993;13(9):3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai H, Kimoto T, Hojo Y, Kawato S, Murakami G, Higo S, … Ogiue-Ikeda M. Modulation of synaptic plasticity by brain estrogen in the hippocampus. Biochimica et Biophysica Acta - General Subjects. 2010;1800(10):1030–1044. doi: 10.1016/j.bbagen.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Mukai H, Tsurugizawa T, Murakami G, Kominami S, Ishii H, Ogiue-ikeda M, … Kawato S. Rapid modulation of long-term depression and spinogenesis via synaptic estrogen receptors in hippocampal principal neurons. Journal of Neurochemistry. 2007;100:950–967. doi: 10.1111/j.1471-4159.2006.04264.x. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development (Cambridge, England) 1992;116(1):201–11. doi: 10.1242/dev.116.1.201. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1483388. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Pinel JPJ. Rhinal Cortex Lesions and Object Recognition in Rats. Behavioral Neuroscience. 1994;108(1):11–18. doi: 10.1037//0735-7044.108.1.11. [DOI] [PubMed] [Google Scholar]
- Navarro-quiroga I, Hernandez-valdes M, Lin SL. Postnatal Cellular Contributions of the Hippocampus Subventricular Zone to the Dentate Gyrus , Corpus Callosum , Fimbria , and Cerebral Cortex. 2006 Feb;845:833–845. doi: 10.1002/cne. 2005. [DOI] [PubMed] [Google Scholar]
- Niu S, Renfro A, Quattrocchi CC, Sheldon M, D’Arcangelo G. Reelin Promotes Hippocampal Dendrite Development through the VLDLR/ApoER2-Dab1 Pathway. Neuron. 2004;41(1):71–84. doi: 10.1016/S0896-6273(03)00819-5. [DOI] [PubMed] [Google Scholar]
- Niu S, Yabut O, D’Arcangelo G. The Reelin Signaling Pathway Promotes Dendritic Spine Development in Hippocampal Neurons. Journal of Neuroscience. 2008;28(41):10339–10348. doi: 10.1523/JNEUROSCI.1917-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander JG, Woolley CS. 17β-Estradiol Acutely Potentiates Glutamatergic Synaptic Transmission in the Hippocampus through Distinct Mechanisms in Males and Females. The Journal of Neuroscience>: The Official Journal of the Society for Neuroscience. 2016;36(9):2677–90. doi: 10.1523/JNEUROSCI.4437-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, … Mikoshibat K. The reeler gene-associated antigen on cajal-retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14(5):899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- Osborn M, Debus E, Weber K. Monoclonal antibodies specific for vimentin. European Journal of Cell Biology. 1984;34(1):137–43. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6428888. [PubMed] [Google Scholar]
- Peters A, Palay S, Webster H. The fine structure of the nervous system. 3. New York: Oxford University Press; 1991. [Google Scholar]
- Pixley SK, de Vellis J. Transition between immature radial glia and mature astrocytes studied with a monoclonal antibody to vimentin. Brain Research. 1984;317(2):201–9. doi: 10.1016/0165-3806(84)90097-x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6383523. [DOI] [PubMed] [Google Scholar]
- Pujadas L, Gruart A, Bosch C, Delgado L, Teixeira CM, Rossi D, … Soriano E. Reelin Regulates Postnatal Neurogenesis and Enhances Spine Hypertrophy and Long-Term Potentiation. Journal of Neuroscience. 2010;30(13):4636–4649. doi: 10.1523/JNEUROSCI.5284-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadros PS, Pfau JL, Goldstein AYN, De Vries GJ, Wagner CK. Sex differences in progesterone receptor expression: a potential mechanism for estradiol-mediated sexual differentiation. Endocrinology. 2002;143(10):3727–39. doi: 10.1210/en.2002-211438. [DOI] [PubMed] [Google Scholar]
- Quadros PS, Pfau JL, Wagner CK. Distribution of Progesterone Receptor Immunoreactivity in the Fetal and Neonatal Rat Forebrain. The Journal of Comparative Neurology. 2007 Dec;56:42–56. doi: 10.1002/cne. 2004. [DOI] [PubMed] [Google Scholar]
- Quadros PS, Schlueter LJ, Wagner CK. Distribution of progesterone receptor immunoreactivity in the midbrain and hindbrain of postnatal rats. Developmental Neurobiology. 2008;68(12):1378–1390. doi: 10.1002/dneu.20664. [DOI] [PubMed] [Google Scholar]
- Quattrocolo G, Maccaferri G. Novel GABAergic circuits mediating excitation/inhibiton of Cajal-Retzius cells in the develping hippocampus. Journal of Neuroscience. 2013;33(413):5486–5498. doi: 10.1016/j.surg.2006.10.010.Use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld P, van Eekelen JAM, Levine S, de Kloet ER. Ontogeny of Corticosteroid Receptors in the Brain. Cellular and Molecular Neurobiology. 1993;13(4):295–319. doi: 10.1007/s00330-011-2364-3. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the Adrenocortical Stress Response: Neuroendocrine Control Mechanisms and the Stress Hyporesponsive Period. Brain Research Reviews. 1986;11:65–76. doi: 10.1007/s00330-011-2364-3. [DOI] [PubMed] [Google Scholar]
- Schlessinger AR, Cowan WM, Gottlieb DI. An Autoradiographic Study of the Time of Origin and the Pattern of Granule Cell Migration in the Dentate Gyrus of the Rat. Journal of Comparative Neurology. 1975;159:149–176. doi: 10.1002/cne.901590202. [DOI] [PubMed] [Google Scholar]
- Sheldon M, Rice DS, Arcangelo GD, Yoneshima H, Nakajima K, Mikoshiba K, … Curran T. Scrambler and yotari disrupt the disabled gene and produce a reeler -like phenotype in mice. Nature. 1997 Oct;389:1995–1998. doi: 10.1038/39601. [DOI] [PubMed] [Google Scholar]
- Simerly RB. WIRED FOR REPRODUCTION: Organization and Development of Sexually Dimorphic Circuits in the Mammalian Forebrain. Annual Review of Neuroscience. 2002;25(1):507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Soriano E, Del Río JA, Martinez A, Supèr H. Organization of the Embryonic and Early Postnatal Murine Hippocampus. I Immunocytochemical Characterization of Neuronal Populations in the Subplate and Marginal Zone. The Journal of Comparative Neurology. 1994;342:571–595. doi: 10.1007/s00330-011-2364-3. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Milner TA, McEwen BS. Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice. Neuroscience. 2008;155(4):1106–1119. doi: 10.1016/j.neuroscience.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. The Medial Temporal Lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nature Reviews. Neuroscience. 2007;8(11):872–83. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Jiam NT, Spiegel AM, Gallagher M. Aging Reduces Total Neuron Number in the Dorsal Component of the Rodent Prefrontal Cortex. Journal of Comparative Neurology. 2012;520(6):1318–1326. doi: 10.1002/cne.22790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supèr H, Martínez A, Del Río JA, Soriano E. Involvement of distinct pioneer neurons in the formation of layer-specific connections in the hippocampus. The Journal of Neuroscience>: The Official Journal of the Society for Neuroscience. 1998;18(12):4616–4626. doi: 10.1523/JNEUROSCI.18-12-04616.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supèr H, Soriano E. The Organization of the Embryonic and Early Postnatal Murine Hippocampus. II. Development of Entorhinal, Commissural, and Septal Connections Studied With the Lipophilic Tracer DiI. The Journal of Comparative Neurology. 1994;344:101–120. doi: 10.1093/pubmed/fdl064. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Namiki J, Shibata S, Mastuzaki Y, Okano H. The Neural Stem/Progenitor Cell Marker Nestin Is Expressed in Proliferative Endothelial Cells, but Not in Mature Vasculature. Journal of Histochemistry & Cytochemistry. 2010;58(8):721–730. doi: 10.1369/jhc.2010.955609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Sokabe M. Bidirectional modulatory effect of 17β-estradiol on NMDA receptors via ERα and ERβ in the dentate gyrus of juvenile male rats. Neuropharmacology. 2013;75:262–273. doi: 10.1016/j.neuropharm.2013.07.029. [DOI] [PubMed] [Google Scholar]
- Tetel MJ, Jung S, Carbajo P, Ladtkow T, Skafar DF, Edwards DP. Hinge and amino-terminal sequences contribute to solution dimerization of human progesterone receptor. Molecular Endocrinology (Baltimore, Md ) 1997;11(8):1114–28. doi: 10.1210/mend.11.8.9963. [DOI] [PubMed] [Google Scholar]
- Traish AM, Wotiz HH. Monoclonal and Polyclonal Antibodies to Human Progesterone Receptor Peptide-(533–547) Recognize a Specific Site In Unactivated (8S) and Activated (4S) Progesterone Receptor and Distinguish between Intact and Proteolyzed Receptors*. Endocrinology. 1990;127(3):1167–1175. doi: 10.1210/endo-127-3-1167. [DOI] [PubMed] [Google Scholar]
- Van Cauter T, Camon J, Alvernhe A, Elduayen C, Sargolini F, Save E. Distinct roles of medial and lateral entorhinal cortex in spatial cognition. Cerebral Cortex. 2013;23(2):451–459. doi: 10.1093/cercor/bhs033. [DOI] [PubMed] [Google Scholar]
- van Eekelen JAM, Bohn MC, de Kloet ER. Postnatal ontogeny of mineralocorticoid and glucocorticoid receptor gene expression in regions of the rat tel- and diencephalon. Developmental Brain Research. 1991;61(1):33–43. doi: 10.1016/0165-3806(91)90111-U. [DOI] [PubMed] [Google Scholar]
- von Haebler D, Stabel J, Draguhn A, Heinemann U. Properties of horizontal cells transiently appearing in the rat dentate gyrus during ontogenesis. Experimental Brain Research. 1992;94(3):33–42. doi: 10.1007/s00330-011-2364-3. [DOI] [PubMed] [Google Scholar]
- Waters EM, Torres-Reveron A, McEwen BS, Milner TA. Ultrastructural localization of extranuclear progestin receptors in the rat hippocampal formation. The Journal of Comparative Neurology. 2008;511(1):34–46. doi: 10.1002/cne.21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss KH, Johanssen C, Tielsch A, Herz J, Deller T, Frotscher M, F??rster E. Malformation of the radial glial scaffold in the dentate gyrus of reeler mice, scrambler mice, and ApoER2/VLDLR-deficient mice. Journal of Comparative Neurology. 2003;460(1):56–65. doi: 10.1002/cne.10644. [DOI] [PubMed] [Google Scholar]
- Willing J, Wagner CK. Progesterone Receptor Expression in the Developing Mesocortical Dopamine Pathway: Importance for Complex Cognitive Behavior in Adulthood. Neuroendocrinology. 2016:1–16. doi: 10.1159/000434725. [DOI] [PMC free article] [PubMed]
- Wilson DIG, Langston RF, Schlesiger MI, Wagner M, Watanabe S, Ainge JA. Lateral entorhinal cortex is critical for novel object-context recognition. Hippocampus. 2013;23(5):352–366. doi: 10.1002/hipo.22095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsky L, Nakata H, Martin BM, Jacobowitz DM. Isolation, partial amino acid sequence, and immunohistochemical localization of a brain-specific calcium-binding protein. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(24):10139–43. doi: 10.1073/pnas.86.24.10139. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2602362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP. The perforant pathg: projections from the entorhinal cortex to the dentate gyrus. Progress in Brain Research. 2007;163:43–61. doi: 10.1016/S0079-6123(07)63003-9. [DOI] [PubMed] [Google Scholar]
- Witter MP, Naber PA, von Haeften T, Machielsen WCM, Rombouts SARB, Barkhof F, … Lopes da Silva FH. Cortico-Hippocampal Communication by Way of Parallel Parahippocampal-Subicular Pathways. Hippocampus. 2000;10:398–410. doi: 10.1680/udap.2010.163. [DOI] [PubMed] [Google Scholar]
- Wu P, Li M shan, Yu D ming, Deng J bo. Reelin, a guidance signal for the regeneration of the entorhino-hippocampal path. Brain Research. 2008;1208:1–7. doi: 10.1016/j.brainres.2008.02.092. [DOI] [PubMed] [Google Scholar]
- Yi SJ, Masters JN, Baram TZ. Glucocorticoid Receptor mRNA Ontogeny in the Fetal and Postnatal Rat Forebrain. Molecular and Cellular Neuroscience. 1994;5:385–393. doi: 10.1006/mcne.1994.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Chai X, Förster E, Frotscher M. Reelin is a positional signal for the lamination of dentate granule cells. 2004:5117–5125. doi: 10.1242/dev.01387. [DOI] [PubMed]
- Zhao S, Förster E, Chai X, Frotscher M. Different signals control laminar specificity of commissural and entorhinal fibers to the dentate gyrus. The Journal of Neuroscience>: The Official Journal of the Society for Neuroscience. 2003;23(19):7351–7357. doi: 10.1523/JNEUROSCI.23-19-07351.2003. http://doi.org/23/19/7351 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]