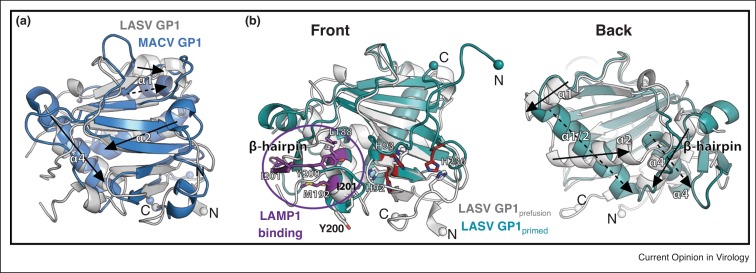
Figure 3.
Comparison of arenavirus GP1 subunits. (a) Structural alignment of LASV GP1 (gray, PDB: 5VK2) and MACV GP1 (blue, PDB: 3KAS). Despite low sequence similarity of 20%, the GP1 subunits of LASV and MACV align with an r.m.s.d. of 2 Å. Major differences map to the loops that connect the β-sheet. For clarity, only the MACV GP1 subunit is shown; other New World arenavirus GP1 subunits align with similar r.m.s.d.s. (b) Overlay of the crystal structures of prefusion GP1, solved at neutral pH (gray, PDB: 5VK2), to the `primed’ GP1 subunit of LASV determined at pH 5 (cyan, PDB: 4ZJF). The histidine triad responsible for sensing pH [37•,43] is shown as ball and stick and is colored gray at neutral pH and red at pH 5. The LAMP1 binding site as it exists at pH 5 is colored purple [38••]. Structural elements of LASV GP1 that vary between the low-pH and neutral-pH forms are indicated. Spheres indicate the N-terminal and C-terminal residues visible in the low-pH GP1 structure and the location of the equivalent residues in the LASV GP trimer structure.