Abstract
The prevalence of Ventricular Assist Device (VAD) therapy has continued to increase due to a stagnant donor supply and growing advanced HF population. We hypothesize that LV size strongly influences biocompatibility and risk of thrombosis. Unsteady computational fluid dynamics (CFD) was used in conjunction with patient-derived computational modeling and virtual surgery with a standard, apically-implanted inflow cannula. A dual focus approach of evaluating thrombogenicity was employed: platelet-based metrics to characterize the platelet environment, and flow-based metrics to investigate hemodynamics. LV end diastolic dimensions (LVEDd) ranging from 4.5 cm – 6.5 cm were studied and ranked according to relative thrombogenic potential. Over 150,000 platelets were individually tracked in each LV model over 15 cardiac cycles. As LV size decreased, platelets experienced markedly increased shear stress histories (SH), while platelet residence time (RT) in the LV increased with size. The complex interplay between increased SH and longer RT has profound implications on thrombogenicity, with a significantly higher proportion of platelets in small LVs having long RT times and being subjected to high SH, contributing to thrombus formation. Our data suggests that small LV size, rather than decreased VAD speed is the primary pathologic mechanism responsible for the increased incidence of thrombosis observed in VAD patients with small LVs.
Keywords: LVAD, Mechanical Circulatory Support, left ventricle size, thrombogenic potential, hemodynamics, Lagrangian metrics, CFD, virtual surgery, shear stress history, residence time
INTRODUCTION
More than 5 million people suffer from heart failure (HF) in the United States alone, and nearly 1 million new cases are diagnosed annually1. Approximately 10% of the HF population in the US progress to medical-therapy refractory advanced HF (Stage D HF)2. The increasing prevalence of Stage D HF, coupled with a stagnant donor supply has resulted in an exponentially-growing demand for left ventricular assist device (LVAD) therapy3,4. While 1 year survival rate for LVAD patients has significantly improved, approaching nearly 90%1,2,5–7, a high risk for devastating complications such as neurologic events and device thrombosis persists1,5,8,9. Thus, there is a strong need for quantitative methods to assess risk factors that influence thrombogenicity of LVAD therapy.
Among anatomic variables with a strong effect on hemodynamics, small left ventricle (LV) size has an important and frequently overlooked influence on LVAD performance and thrombogenicity. This may occur in HF patients with natively small LVs, as well as patients whose LV reverse remodels with mechanical unloading during LVAD support. As HF prevalence has increased, so has the number of patients with relatively small LV diameters. However, assessment of biocompatibility has yet to be performed for this cohort.10–12.
Thrombus initiation is profoundly impacted by hemodynamics; platelets sense their microenvironment and initiate the coagulation cascade under certain mechanical and chemical conditions13,14. Unfavorable intraventricular hemodynamics such as stasis and high shear exposure zones activate platelets traversing the LV, leading to thrombosis or cerebrovascular events8,15–17. Anecdotal evidence has pointed to LV size as a possible risk factor for LVAD thrombosis10,12,18. However, the influence of LV size on LVAD performance and thrombogenicity has not formally been quantified. Assessing thrombogenicity entails elucidating the platelet microenvironment, i.e. a Lagrangian approach that tracks and explores platelet activation via shear stress exposure and potential for blood stasis 19,20.
We hypothesize that smaller LV volumes contribute to unfavorable hemodynamics, adversely influencing thrombogenicity of LVAD therapy. We use a combination of patient-derived anatomical models, virtual surgery and unsteady CFD to model intraventricular hemodynamics. Using our previously published methodology21,22, we compute platelet-environment-based thrombogenic indices platelet RT and SH to quantify the impact of LV size on thrombogenicity of LVAD therapy. Our methodology is clinically generalizable and employs a device-neutral approach to incorporate LV size considerations to evaluate and optimize VAD therapy.
METHODS
Blood flow in the LV is simulated within 3D patient-derived models of an average (6.5 cm) and small-sized (4.5 cm) LV implanted with an LVAD inflow cannula via virtual surgery.
Virtual surgery
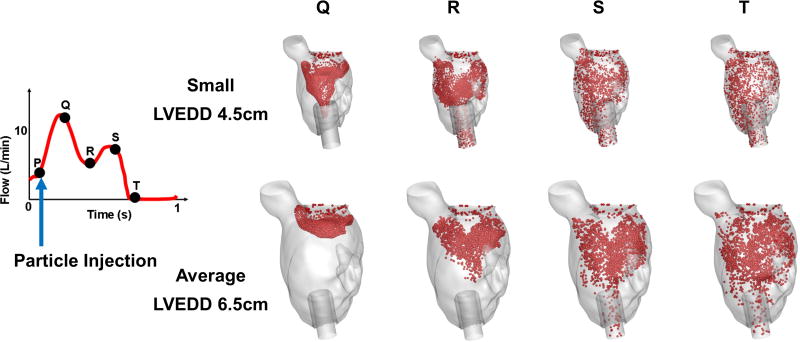
A patient-derived anatomical LV model was created by image segmentation of computed tomography (CT) images (70kg M) with an idiopathic, non-ischemic, dilated cardiomyopathy (left-ventricular end-diastolic diameter (LVEDd) of 6.5cm). To preserve fidelity with a small LV without inadvertently introducing confounding anatomic variables, the LV was scaled down geometrically to create a model with an LVEDd of 4.5cm. Apical cannulation was performed in standard surgical fashion with a 27mm inflow cannula, consistent with contemporary clinical practice. (Figure 1).
Figure 1.
Intraventricular blood flow dynamics highlighted by transport of particles injected at the same point in the cardiac cycle (point P) for both small (4.5 cm) and average (6.5 cm) LV models
Computational model
Unsteady computational fluid dynamics (CFD) was employed to directly simulate intraventricular hemodynamics. Blood was modeled as a homogeneous, Newtonian fluid using Navier-Stokes equations to simulate intraventricular hemodynamics using high temporal and spatial resolution to capture chaotic flow and development of instabilities.
We simulated the motion of platelet surrogate particles to obtain information about the platelet microenvironment. 3 micron diameter neutrally-buoyant platelet surrogate particles (massless flow tracers) were injected into the LV through the MV every 1/10th of a second for 10 seconds, and individually tracked over 15 seconds to evaluate thrombogenic indices (platelet residence time (RT), shear stress history (SH) exposure and shear accumulation). Over 150,00 particles are individually tracked for each case and particle trajectories were constructed as they traverse the LV. The particle residence time (RT) was calculated by tracking the time each particle remained in the vascular domain:
Where i is an index for each particle, represents the time the particle is injected into the domain, and represents the time the particle trajectory ends as a particle exits the domain or the simulation is terminated. While many factors influence platelet activation, one of the most widely accepted theories is shear-induced platelet-activation (SIPA). Lagrangian tracking allows for determination of accumulated shear stress on each platelet, as a function of time in the flow, to evaluate the level of SIPA associated with each LV size studied:
Where τ is the instantaneous shear stress magnitude at time t′ and X(t′) is the platelet’s location at that time. For more details about the numerical models, please refer to our previous work21,22. A patient-derived unsteady flow waveform was applied at the mitral valve (MV) to achieve a time-averaged flow of 5 L/min for both models. The aortic valve (AV) was assumed closed for the duration of the simulations to replicate full LVAD support.
Global (Eulerian) hemodynamic parameters such as wall shear stress (WSS) were measured and incorporated with local (Lagrangian), particle-based metrics such as RT and SH in evaluating the impact of LV size on thrombogenicity.
Quantifying thrombogenic potential
Thrombogenic potential (TP) for each LV model was quantitatively evaluated based on ensemble platelet SH and RT using a modified formula of our previously published thrombogenicity index21.
RESULTS
CFD simulations were performed for both LV models, covering 15 consecutive cardiac cycles. A time varying (unsteady) flow profile was specified at the MV, and data was collected and used after the initial transients (3 seconds) subsided and statistical periodicity was achieved.
Blood flow patterns
Intraventricular hemodynamics differed markedly between the average-sized and small LVs. Blood flow velocity was higher in the small LV owing to the reduced cross-sectional area through which the same LVAD input has to flow. As a result, blood entering the LV via the MV began to exit through the LVAD inflow cannula after 0.25 seconds in the smaller (4.5 cm) ventricle, compared to ~0.5 seconds in the average-sized (6.5 cm) LV (Figure 1).
There are stagnation zones in both the average-sized and small LV, primarily in the subaortic region. Other areas of stasis were found in the narrow spaces between the inflow cannula and the LV apex.
Blood-suspended platelet metrics
Over 150,000 platelet-surrogate particles were individually tracked for 15 seconds to obtain detailed metrics such as RT and SH. These metrics are utilized in quantifying thrombogenic indices for each LV model.
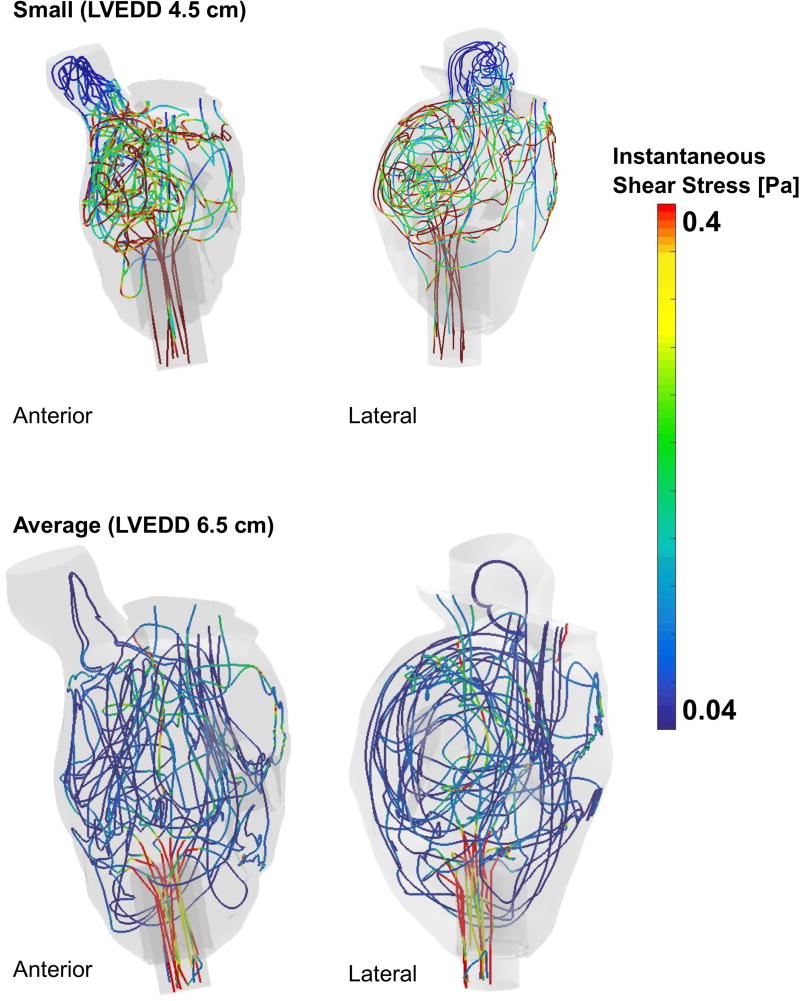
Platelet trajectories reveal complex intraventricular paths taken by blood traversing the LV. Both the average and small LV show circuitous paths, with platelets traveling through potential stasis zones near the closed AV and ventricular apex, recirculating around the LV multiple times before finally entering the LVAD inflow cannula. Owing to the unsteady CFD simulation and the non-uniform intraventricular flow, local shear stresses vary temporally and spatially everywhere in the LV. This is evident in Figure 2, where randomly chosen particle trajectories are plotted for both LV models. This demonstrates the spatio-temporal variation of shear stresses by plotting the instantaneous local shear stress on representative platelet trajectories as they traverse the ventricle, and enables investigation of the regions where a platelet experiences locally high shear stresses along its trajectory. Platelets in the smaller LV experience higher instantaneous shear due to high velocity blood flow.
Figure 2.
Representative platelet trajectories depicting the instantaneous shear that they experience as they traverse the LV for both small (4.5 cm) and average (6.5 cm) models
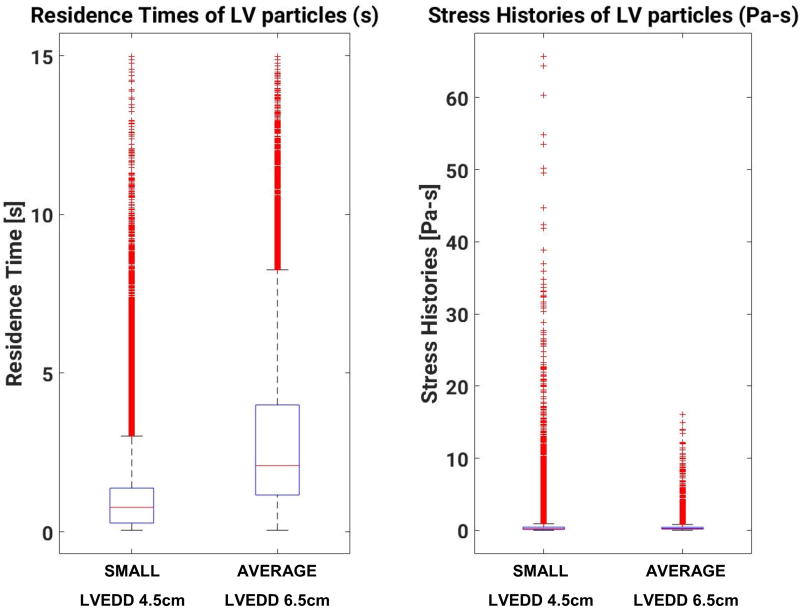
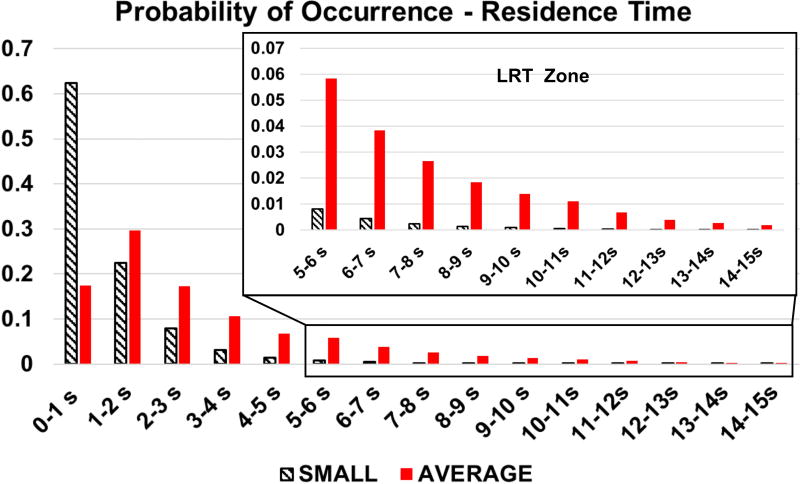
Figure 3 shows boxplots of RT and SH statistics for all platelets injected at the MV for the average and small LV models. Owing to the higher flow velocity in the smaller (4.5 cm) LV, particles traverse the smaller ventricles about 150% faster than in the average-sized (6.5 cm) ventricle. The mean RT is about one second lower in the smaller LV than in the average-sized LV. The tails of the distribution, however, are extremely long (Figure 4), with platelets that remain in the LV for the duration of the simulation (>15 seconds) in both cases. Forced by the slower mean velocity, platelets in the average-sized (6.5 cm) LV have an increased likelihood of having Long Residence Times (LRT).
Figure 3.
Boxplots of particle RT and SH for both small (4.5 cm) and average (6.5 cm) LV models
Figure 4.
Probability of occurrence of particle RT in both small (4.5 cm) and average (6.5 cm) LV models
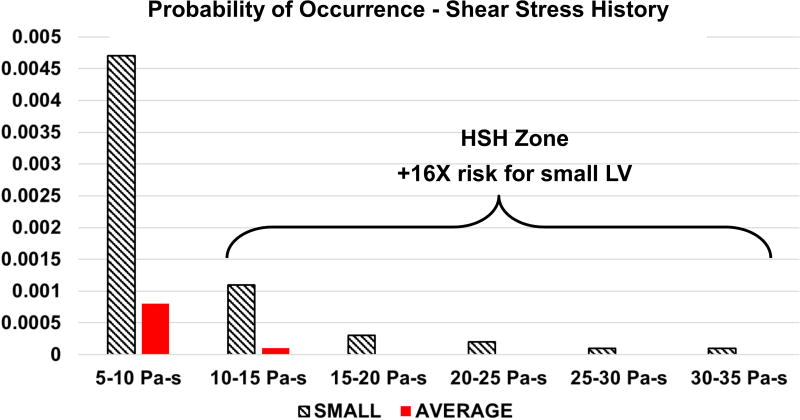
The median SH accumulated by platelets was largely insensitive to LV size (0.23 Pa-s for the small vs 0.26 Pa-s for the average size). Platelet distribution of SH in the small LV had a much longer tail, with values up to 65.68 Pa-s. This represents a 309% increase over the maximum SH experienced by platelets in the average-sized LV (16.05 Pa-s). If the threshold for high shear history (HSH) is set at 10 Pa-s, more platelets in the small (4.5 cm) LV are found to be in the HSH exposure zone (Figure 5), with an increased likelihood (16-fold) compared to the average-sized (6.5 cm) ventricle.
Figure 5.
Probability of occurrence of particle SH for both small (4.5 cm) and average (6.5 cm) LV models
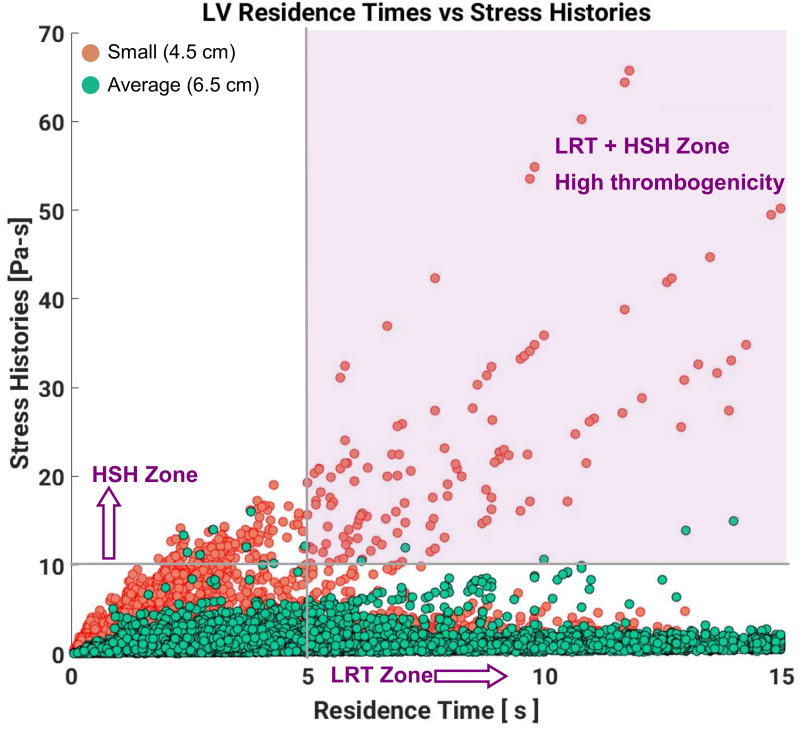
The interdependence of platelet exposure to high SH and their RT can be seen in Figure 6. Most platelets with high SH in the small LV accumulate the high SH value very early after injection into the LV, as evidenced by the high slope cluster of the orange dots. This behavior is qualitatively different for the average-sized LV, where most platelets exposed to high SH are those with high RT, lingering in the LV and accumulating medium levels of shear stress for longer periods of time. As mentioned earlier, we can identify a LRT zone and a HSH zone using arbitrary thresholds of 5s for RT and 10 Pa-s for SH. The intersection of these two zones (i.e. LRT + HSH zones) in the upper right quadrant indicates a high thrombogenicity (HT) zone, where platelets not only spend long times in the LV but also accumulate high shear. Significantly more platelets from the small LV (9.28%) occur in this zone compared to the average-sized LV (0.32%), revealing a higher thrombogenic potential for the small LV.
Figure 6.
Interdependence of platelet RT and SH behavior for both small (4.5 cm) and average (6.5 cm) LV models
Evaluation of thrombogenic potential
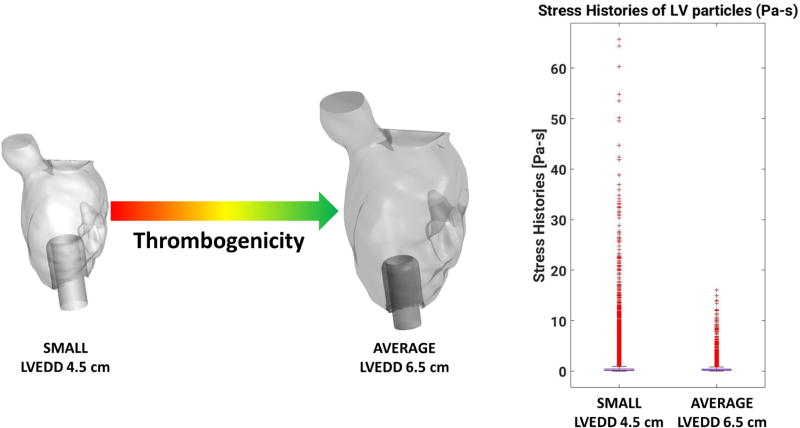
Composite TP was evaluated for both LV sizes using a modified formula of our previously published metric21, which enabled incorporation of the percentage of particles in the HT zone in figure 6. Briefly, various statistical descriptors of platelet RT and SH distributions are combined into a single metric to quantify the relative TP of LVAD support of both a small (4.5 cm) and average-sized (6.5 cm) LV. LVAD support was demonstrated to be more thrombogenic for the smaller LV with a TP score of 0.80, mainly due to unfavorable hemodynamics and an increased probability of platelets being exposed to high shear, initiating activation and aggregation. Note that the TP score is relative; i.e. a TP score of 0 does not indicate absence of thrombogenicity, it only indicates lower thrombogenicity compared to the other case studied.
DISCUSSION
Cerebrovascular events and thrombosis remain the most devastating complications of LVAD therapy3,5,23,24. As LVAD therapy continues to increase in prevalence, risk factors for poor outcomes need to be evaluated closely, including anatomic variables such as LV size 16,25–27. Investigations of intraventricular flow are limited28–30, with the impact of LV size on thrombogenicity still unexplored. Observational evidence of the influence of LV size on adverse events10,12,31 has not been mechanistically confirmed. Interdependencies between intraventricular flow, LV size and LVAD speed complicates assessment of thrombogenicity. Using Lagrangian analysis of platelet trajectories inside patient-derived LV models with different sizes, we demonstrate that, for a similar blood flow rate, smaller intraventricular volumes are associated with a significantly larger proportion of platelets subjected to both high RT and accumulating high SH, indicating elevated thrombogenic risk. This has profound clinical implications, highlighting the need to consider the complex interplay of VAD speed, patient MAP and LV size in clinical decision-making. In summary, it is imperative to dismiss the “one size fits all” approach in favor of a multidimensional clinical strategy of patient care.
Using computational modeling and virtual surgery, we created two geometrically-similar LV size models - a small LV with an LVEDd of approximately 4.5 cm and an average-sized model with an LVEDd of approximately 6.5 cm. LVAD support in patients with small intraventricular volumes (irrespective of etiology), results in markedly different hemodynamics compared to average-sized ventricles, strongly influencing biocompatibility. Consequently, small LV geometry occurring natively, or with reverse remodeling with LVAD unloading has major clinical implications.
For a given flow rate, blood transits a small (4.5cm) LV much faster than an average-sized (6.5 cm) LV. In our study, a nominal 5 L/min enters the LV through the MV with a closed AV, modeling full VAD support. As a result, all blood entering the LV exited via the LVAD inflow cannula. Median RT of platelets for the small LV was 0.77 seconds, nearly three times lower than the 2.1 seconds RT in the average-sized LV. However, the tails of the distributions of RT are extremely long for both cases, with platelets remaining in the LV for the duration of the simulation (>15 s). Blood trapped in areas of stagnation such as proximal to the AV and the narrow space between the inflow cannula and the LV apex is a necessary condition for platelet aggregation, especially if they have been activated before lodging in the high RT regions, and act as a nidus for thrombus formation.
Intraventricular hemodynamics exacerbate thrombogenicity of LVAD therapy via shear induced platelet activation (SIPA)20,32. This mode of platelet activation is distinct from an unfavorable environment inside the LVAD, as platelets exposed to detrimental hemodynamic environments in the ventricle initiate the process of activation and aggregation prior to entering the LVAD. When this occurs, platelets are predisposed to form micro-thrombi. In our comparative study, despite flow transiting the smaller LV in a shorter time on average, platelets were exposed to significantly higher cumulative levels of shear. The maximum SH in the small ventricle (65.68 Pa-s) was more than three times the value for the average-sized ventricle (16.05 Pa-s). Statistically, the probability that a platelet would experience very high SH (>10 Pa-s) in the small ventricle is 16 times higher than in the average-sized LV. Moreover, platelet dynamics in the small ventricle are qualitatively different from the average-sized LV model, as observed in the SH versus RT scatter plot in Figure 5. Platelets that reach high values of SH in the small LV do so by being exposed to relatively high values of shear stress (> 5 Pa-s) over their short RT in the ventricle. This is in contrast with the high SH platelets in the average-sized ventricle, where they are exposed to above-average but still moderate (< 2 Pa-s) values of shear stress, but for an extended period of time. This novel mechanism, which has not been described to date, allows platelets to reach high SH even when the median RT is low. Conversely, some platelets recirculate within the domain for extended periods of time but do not accumulate high SH. The high-risk platelets not only spend longer times in the domain, but also reach pathologically high levels of SH exposure (> 10 Pa-s), which continues to increase for those platelets recirculating in the domain. Additional cardiac cycles in the simulation only confirm that these platelets are subject to an extreme hemodynamic environment, consistent with a significant increase in the risk of initiation of platelet activation and aggregation via SIPA for patients with smaller LVEDd.
Despite great advancements in LVAD therapy over the past decade, HF patients with small ventricles continue to experience more adverse events and complications than patients with average sized ventricles. Several studies have reported increased incidence of stroke, bleeding, and hemolysis12,31,33,34, in addition to impaired long-term survival of small-ventricle patients implanted with an LVAD35,36,37. In a recent study, patients with a smaller LV had a higher incidence of stroke and other adverse events compared to patients with average to larger LV10. Moreover, another recent study confirmed that patients with small pre-implant ventricles (< 5cm) had significantly higher mortality and were associated with far worse outcomes, compared to patients with average sized LVs18. The impact of unfavorable hemodynamics in the LV increases as the ventricle undergoes reverse remodeling in response to LVAD therapy. These studies demonstrate that the intraventricular hemodynamics resulting from LVAD implantation in small ventricles needs further investigation and optimization.
Surgical implantation technique, pump speed and patient management need to be carefully considered for small ventricle patients. Our study indicates that intraventricular hemodynamics have a strong influence on thrombogenic indices, which is summarized graphically in figure 7. When analyzed for a similar blood flow rate (5 L/min), smaller intraventricular volumes are associated with lower median RT, but a high percentage of long-RT outliers, and to higher shear rates due to the higher velocities through smaller cross-sectional areas. While there is a commonly held misconception that to decrease the probability of thrombosis, clinicians should err on the side of higher VAD speeds, it is clear that small LV size, rather than decreased VAD speed is primarily responsible for the observed increased risk of thrombosis. It is thus clear that if LVAD speed is not decreased accordingly in patients with small LVs, it will further exacerbate thrombogenic hemodynamics, increasing stroke risk.
Figure 7.
Summary graphic indicating thrombogenicity variation according to LV size
Limitations
The AV region is an area of stagnation as we assumed a closed AV. The rationale for this simplification is (i) the focus of our simulations is near the LV apex, and (ii) AV opening dynamics and its influence on ventricular and aortic hemodynamics are out of the scope of the current paper, but have been analyzed in another study that builds on our previous study of the beneficial effects of AV opening21 . The LV walls were considered rigid, simplifying the dynamics of flow in the LV. An analysis of ventricular wall motion from echo-Doppler revealed minimal changes of the ventricular wall between systole and diastole. Platelets were assumed to have purely elastic collisions in relation to the ventricular walls. Future models should incorporate inter-platelet signaling and adhesion models.
CONCLUSIONS
Our study demonstrates that LV hemodynamics differ markedly based on LV chamber size. In VAD patients with small LVs, platelets gravitate towards the high thrombogenic (LRT+HSH) zone very quickly, thus, being subjected to pathologically high SH, leading to platelet activation and aggregation, resulting in thrombus formation. This markedly increases the risk of device thrombosis and neurologic events for VAD patients with small LVs, or patients whose LVEDd decreases due to reverse remodeling on support. This should be taken into account for patient selection, device management and anticoagulation strategies. Small LV chamber size, rather than decreased VAD speed, is primarily responsible for the clinical observation of excess thrombosis in patients with small LVs.
Acknowledgments
This work was funded in part by an American Heart Association (AHA) (postdoctoral fellowship 16POST30520004).
The authors would like to thank the High Performance Computing (HPC) group for providing the high performance computing facility (Hyak) at the University of Washington to conduct the simulations described in this study. Thanks to Pablo Martinez-Legazpi and Juan Carlos del Alamo for the MV flow rate waveform and the quantification of restricted ventricular volume change over the cardiac cycle. The authors would also like to thank the reviewers for their time and valuable suggestions for improving the manuscript.
Abbreviations
- LV
Left Ventricle
- LVAD
Left Ventricular Assist Device
- CFD
Computational Fluid Dynamics
- MV
Mitral Valve
- AV
Aortic Valve
- RT
Residence Time
- LRT
Long Residence Time
- SH
Shear Stress History
- HSH
High Shear History
- HT
High Thrombogenicity
- TP
Thrombogenic Potential
- SIPA
Shear Induced Platelet Activation
Footnotes
Disclosures:
CM has consulting relationships with Abbott (Thoratec), Medtronic (HeartWare) and Abiomed and is an investigator for Abbott (Thoratec), Medtronic (HeartWare), and SynCardia. JB has consulting relationships with Abbott (Thoratec), Medtronic (HeartWare) and Abiomed. NAM has consulting relationships with Abbott (Thoratec) and Medtronic (HeartWare), and is an investigator for Abbott (Thoratec), Medtronic (HeartWare), and SynCardia.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mancini D, Colombo PC. Left Ventricular Assist Devices: A Rapidly Evolving Alternative to Transplant. J Am Coll Cardiol. 2015;65:2542–55. doi: 10.1016/j.jacc.2015.04.039. [DOI] [PubMed] [Google Scholar]
- 3.Slaughter MS, Rogers JG, Milano Ca, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 4.Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 5.Najjar SS, Slaughter MS, Pagani FD, et al. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2014;33:23–34. doi: 10.1016/j.healun.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Jorde UP, Kushwaha SS, Tatooles AJ, et al. Results of the Destination Therapy Post-FDA-Approval Study with a Continuous Flow Left Ventricular Assist Device: A Prospective Study Using the INTERMACS Registry. J Am Coll Cardiol. 2014;63 doi: 10.1016/j.jacc.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 7.Mehra MR, Naka Y, Uriel N, et al. A Fully Magnetically Levitated Circulatory Pump for Advanced Heart Failure. N Engl J Med. 2017;376:440–450. doi: 10.1056/NEJMoa1610426. [DOI] [PubMed] [Google Scholar]
- 8.Mehra MR, Stewart GC, Uber Pa. The vexing problem of thrombosis in long-term mechanical circulatory support. J Hear Lung Transplant. 2014;33:1–11. doi: 10.1016/j.healun.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Starling RC, Moazami N, Silvestry SC, et al. Unexpected Abrupt Increase in Left Ventricular Assist Device Thrombosis. N Engl J Med. 2014;370:33–40. doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
- 10.Shah P, Birk S, Maltais S, et al. Left ventricular assist device outcomes based on flow configuration and pre-operative left ventricular dimension: An Interagency Registry for Mechanically Assisted Circulatory Support Analysis. J Hear Lung Transplant. 2016:1–10. doi: 10.1016/j.healun.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Lee S, Katz JN, Jorde UP, et al. Outcomes of Adult Patients with Small Body Size Supported with a Continuous-Flow Left Ventricular Assist Device. ASAIO J. 2016;62:646–651. doi: 10.1097/MAT.0000000000000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zafar F, Villa CR, Morales DL, et al. Does Small Size Matter With Continuous Flow Devices?: Analysis of the INTERMACS Database of Adults With BSA ≤1.5 m(2) JACC Heart Fail. 2016;5:123–131. doi: 10.1016/j.jchf.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Para a, Bark D, Lin a, Ku D. Rapid platelet accumulation leading to thrombotic occlusion. Ann Biomed Eng. 2011;39:1961–1971. doi: 10.1007/s10439-011-0296-3. [DOI] [PubMed] [Google Scholar]
- 14.Xenos M, Girdhar G, Alemu Y, et al. Device Thrombogenicity Emulator (DTE)--design optimization methodology for cardiovascular devices: a study in two bileaflet MHV designs. J Biomech. 2010;43:2400–9. doi: 10.1016/j.jbiomech.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.May-Newman K, Enriquez-Almaguer L, Posuwattanakul P, Dembitsky W. Biomechanics of the aortic valve in the continuous flow VAD-assisted heart. ASAIO J. 2010;56:301–308. doi: 10.1097/MAT.0b013e3181e321da. [DOI] [PubMed] [Google Scholar]
- 16.Blitz A. Pump thrombosis — A riddle wrapped in a mystery inside an enigma. Ann Cardiothorac Surg. 2014;2:450–471. doi: 10.3978/j.issn.2225-319X.2014.09.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burkhoff D, Sayer G, Doshi D, Uriel N. Hemodynamics of mechanical circulatory support. J Am. 2015 doi: 10.1016/j.jacc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Patel SR, Saeed O, Naftel D, et al. Outcomes of Restrictive and Hypertrophic Cardiomyopathies after LVAD: an INTERMACS Analysis. J Card Fail. 2017;23:859–867. doi: 10.1016/j.cardfail.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Sheriff J, Soares JS, Xenos M, Jesty J, Bluestein D. Evaluation of shear-induced platelet activation models under constant and dynamic shear stress loading conditions relevant to devices. Ann Biomed Eng. 2013;41:1279–1296. doi: 10.1007/s10439-013-0758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramstack JM, Zuckerman L, Mockros LF. Shear-induced activation of platelets. J Biomech. 1979;12:113–125. doi: 10.1016/0021-9290(79)90150-7. [DOI] [PubMed] [Google Scholar]
- 21.Mahr C, Chivukula VK, McGah P, et al. Intermittent Aortic Valve Opening and Risk of Thrombosis in VAD Patients. ASAIO J. 2017;63:425–432. doi: 10.1097/MAT.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aliseda A, Chivukula V, Mcgah P, et al. LVAD outflow graft angle and thrombosis risk. ASAIO J. 2017;63:14–23. doi: 10.1097/MAT.0000000000000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aaronson KD, Slaughter MS, Miller LW, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012;125:3191–3200. doi: 10.1161/CIRCULATIONAHA.111.058412. [DOI] [PubMed] [Google Scholar]
- 24.Upshaw JN, Kiernan MS, Morine KJ, Kapur NK, DeNofrio D. Incidence, Management, and Outcome of Suspected Continuous-Flow Left Ventricular Assist Device Thrombosis. ASAIO J. 2016;62:33–39. doi: 10.1097/MAT.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 25.Bartoli CR, Ailawadi G, Kern JA. Diagnosis, Nonsurgical Management, and Prevention of LVAD Thrombosis. J Card Surg. 2014;29:83–94. doi: 10.1111/jocs.12238. [DOI] [PubMed] [Google Scholar]
- 26.Guglin M. What did we learn about VADs in 2016? VAD J. 2017;3 [Google Scholar]
- 27.Fatullayev J, Samak M, Sabashnikov A, et al. Continuous-Flow Left Ventricular Assist Device Thrombosis: A Danger Foreseen is a Danger Avoided. Med Sci Monit Basic Res. 2015;21:141–4. doi: 10.12659/MSMBR.894840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fraser KH, Taskin ME, Griffith BP, Wu ZJ. The use of computational fluid dynamics in the development of ventricular assist devices. Med Eng Phys. 2011;33:263–280. doi: 10.1016/j.medengphy.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ong C, Dokos S, Chan B, et al. Numerical investigation of the effect of cannula placement on thrombosis. Theor Biol Med Model. 2013;10:35. doi: 10.1186/1742-4682-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laumen M, Kaufmann T, Timms D, et al. Flow Analysis of Ventricular Assist Device Inflow and Outflow Cannula Positioning Using a Naturally Shaped Ventricle and Aortic Branch. Artif Organs. 2010;34:798–806. doi: 10.1111/j.1525-1594.2010.01098.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee S, Katz JN, Jorde UP, et al. Outcomes of Adult Patients with Small Body Size Supported with a Continuous-Flow Left Ventricular Assist Device. ASAIO J. 2016;62:646–651. doi: 10.1097/MAT.0000000000000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Einav S, Bluestein D. Dynamics of blood flow and platelet transport in pathological vessels. Ann N Y Acad Sci. 2004;1015:351–66. doi: 10.1196/annals.1302.031. [DOI] [PubMed] [Google Scholar]
- 33.Hsich EM. Does Size Matter With Continuous Left Ventricular Assist Devices? JACC Hear Fail. 2017;5:123–131. doi: 10.1016/j.jchf.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Komoda T, Drews T, Hetzer R, Lehmkuhl HB. Lower body surface area is highly related to mortality due to stroke or systemic bleeding in patients receiving an axial flow blood pump as a left ventricular assist device. Eur J Cardio-Thoracic Surg. 2013;43:1036–1042. doi: 10.1093/ejcts/ezs483. [DOI] [PubMed] [Google Scholar]
- 35.Kawabori M, Kurihara C, Sugiura T, et al. Effect of Preoperative Small Left Ventricle on Patients with Chronic Heart Failure Undergoing Implantation of Long-Term Continuous Flow Ventricular Assist Devices: Comparative Analysis of HeartMate II and HeartWare Devices. J Hear Lung Transplant. 2017;36:S340–S341. [Google Scholar]
- 36.Komoda T, Drews T, Hetzer R, Lehmkuhl HB. Adult candidates for heart transplantation with larger body surface area have better prognosis on waiting list after progression to critically ill status. Eur J Cardio-Thoracic Surg. 2011;39:317–322. doi: 10.1016/j.ejcts.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 37.Ibrahim M, Kilic A, Atluri P. Left Ventricular Assist Devices and Small Body Surface Area – A Clinical Concern? Circ J. 2016;80:1901–1902. doi: 10.1253/circj.CJ-16-0749. [DOI] [PubMed] [Google Scholar]