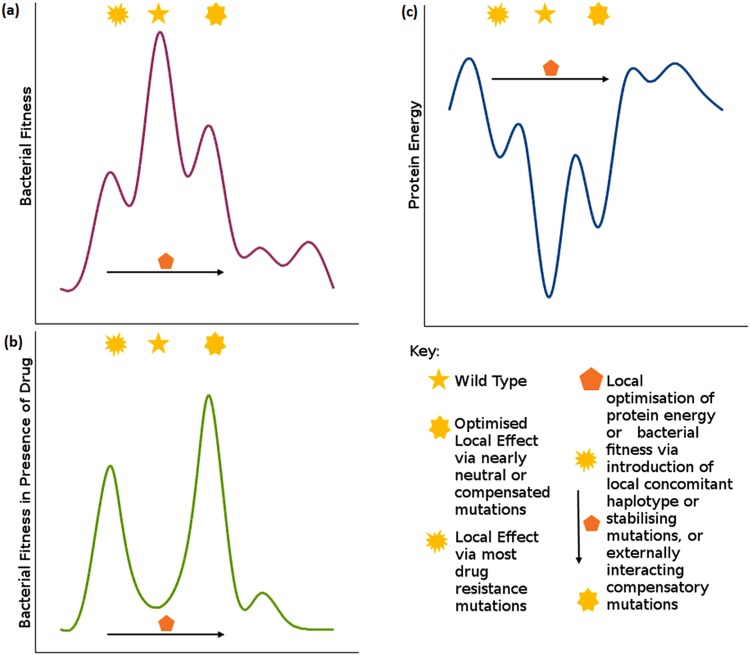
Figure 4.
A theoretical bacterial fitness plot in the absence (a) and presence (b) of drug, running in parallel with a theoretical protein energy plot (c) showing different levels of protein minima. Changes in bacterial fitness in the presence of the drug is approximated by the changes clinically observed to susceptibility and supported, where available, by experimentally measured minimum inhibitory concentration (MIC) values from susceptible to degrees of resistance. The ‘Global Minimum’ refers to the overall energy of the protein. This minimum is thought to be higher in the mutant protein when compared to the wildtype, resulting in a lower protein fitness. However, the mutant protein confers a higher cellular fitness to that of the wildtype when subjected to drug selection pressure. (b) ‘Local Minimum’ refers to the local changes imparted by low frequency mutations, while ‘Optimised Local Minimum’ is an improved local minimum brought about by high frequency mutations, or concomitant mutations. As an example of values that can attributed to this schematic e.g. katG mutations R463L and S315T: the change in protein energy is −0.215 and −0.154 kcal/mol respectively; the change in bacterial fitness in the presence of the drug is 0.15 and 2.7 respectively as measured by MIC.