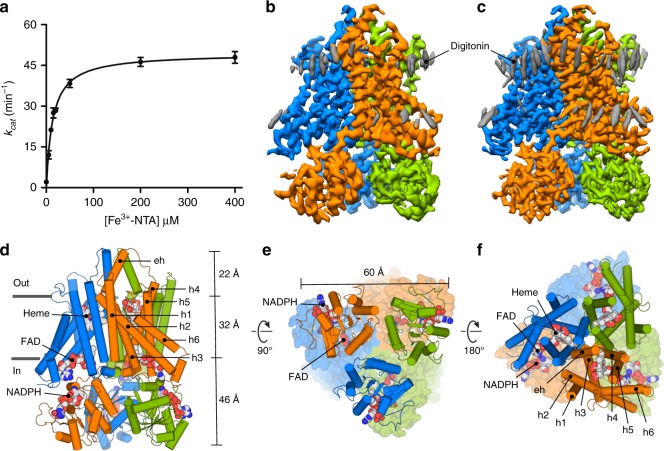
Fig. 1.
Catalytic activity and molecular architecture of human STEAP4. a Ferric-reductase activity of detergent-purified STEAP4EM with varying Fe3+-NTA concentrations. STEAP4EM exhibits a KM of 13.7 ± 0.8 µM for Fe3+-NTA and a kcat of 49.4 ± 0.8 min−1. Error bars represent the standard deviation between triplicate measurements. b Sharpened density map of the cofactor-bound dataset at 3.8-Å resolution colored by chain. c Sharpened density map of the cofactor/substrate-bound dataset at 3.1-Å resolution colored by chain. The grey densities do not exhibit protein features but likely represent the sterol moiety of digitonin. d–f Trimeric arrangement of STEAP4, viewed parallel to the membrane as a sideview (d) and perpendicular to the membrane from the cytoplasm (e), and extracellular milieu (f). For the cytoplasmic and extracellular views, the domain in the background is shown as surface