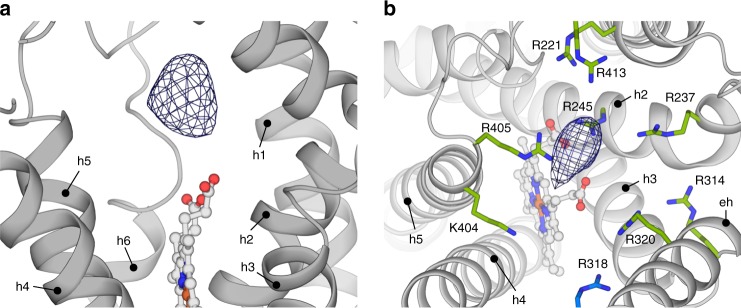
Fig. 4.
Substrate-binding site above the porphyrin. a Sideview of the extracellular side of the TMD of a STEAP4 protomer (shown in grey). The difference density is contoured at 18σ. b Positively charged amino acid ring that surrounds the substrate density, as viewed from the extracellular side of the membrane. Residue R318 belongs to an adjacent STEAP4 protomer. The difference density is contoured at 18σ. The shown residues display variable conservation as basic amino acids (percentage in brackets) throughout 607 STEAP orthologs:15 R221 (96%), R237 (87%), R245 (72%), R314 (98%), R318 (97%) R320 (48%), K404 (48%), R405 (94%), R413 (72%)