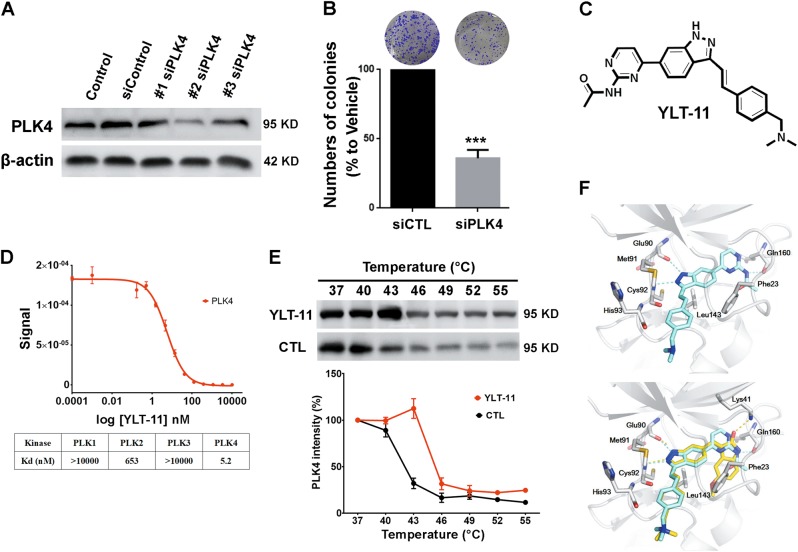
Fig. 1. The activity of PLK4 could be potently inhibited by YLT-11, a novel and specific PLK4 inhibitor.
a MDA-MB-231 transfected with PLK4 siRNA. The expression of PLK4 was determined by Western blotting. b The number of colonies in MDA-MB-231 transfected with PLK4 siRNA. Quantification was shown in the lower panel. Data are expressed as mean ± SD for three independent experiments. Columns, mean, bars, SD (***p < 0.001 vehicle control). c Chemical structure of YLT-11. d Binding assays for YLT-11–PLK4 interaction. Upper panel: Determination of quantitative binding constants of PLK4; lower panel: binding constants of PLK family. e Cellular thermal shift assay from 40 to 55 ℃ of MDA-MB-231 lysates with or without YLT-11 incubation. The image (upper panel) and quantification of the band intensities (lower panel) in immunoblotting. Graphic data were run in triplicate and shown as the mean ± SD. f YLT-11 is docked into the active site of PLK4, showing interactions between YLT-11 and PLK4 in the three-dimentional structure, and YLT-11 binds to PLK4 via a mode highly similar to that of the inhibitor 400631