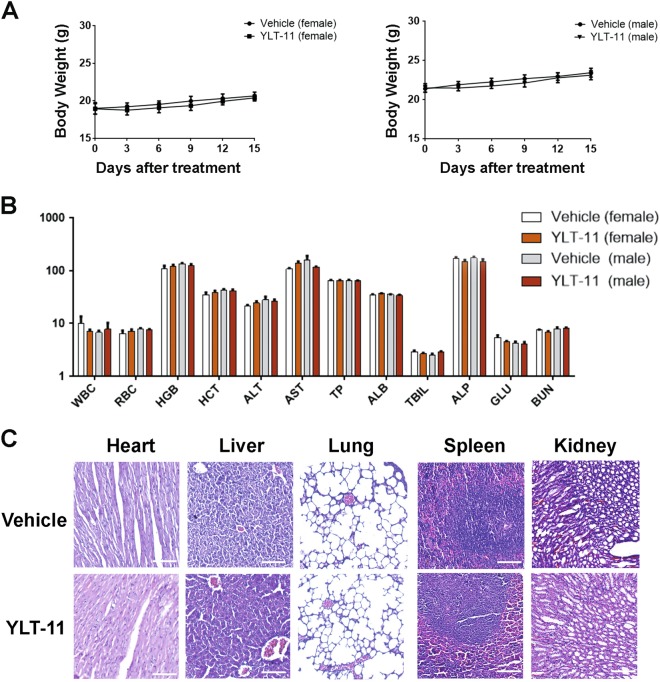
Fig. 7. Preliminary safety evaluation of YLT-11 in BALB/c mice.
a The difference of body weights between two administrated groups (female and male mice) and two vehicle groups (female and male mice) were not significant. Data are expressed as mean ± SD (n = 5). b Hematological and serum biochemical values of mice at day 14 (n = 5) for both vehicle and treated groups. Units of the parameters are as follows: WBC white blood cell (109/L); RBC red blood cell (1012/L); HGB hemoglobin (g/L); ALB albumin and TP total protein (g/L); ALT alanine transarninase (U/L); AST aspartate aminotransferase (U/L); TBIL total bilirubin (μmol/L); ALP alkaline phosphatase (U/L); BUN blood urea nitrogen; and GLU glucose (mM). c YLT-11 did not cause obvious pathologic abnormalities in normal tissues. Paraformaldehyde-fixed organs (heart, liver, spleen, lungs, and kidneys) were processed for paraffin embedding and then stained by hematoxylin and eosin. Images shown are representatives from each group. Scale bars, ×20 for micrograph