Abstract
Background
Oncofertility is a crucial facet of cancer supportive care. The publication of guidelines for the cryopreservation of oocytes and ovarian tissue is becoming increasingly prevalent in Japan and an updated overview is necessary.
Methods
In order to provide an updated overview of oncofertility care, original research and review articles were searched from the PubMed database and compared in order to present clinical care in Japan.
Results
In Western countries, various methods for ovarian stimulation, such as the combined use of aromatase inhibitors and random‐start protocols, have been reported. Although ovarian tissue cryopreservation, mainly performed via the slow‐freezing method, also has yielded >100 live births, the optimal indications and procedures for the auto‐transplantation of cryopreserved tissue have been under investigation. In Japan, however, vitrification is prevalent for ovarian tissue cryopreservation, although its efficacy has not yet been established. The quality of network systems for providing oncofertility care in Japan varies greatly, based on the region.
Conclusion
There remain many issues in the optimization of oncofertility care in Japan. Along with the regional oncofertility networks, the creation of “oncofertility navigators” from healthcare providers who are familiar with oncofertility, such as nurses, psychologists, and embryologists, could be useful for supplementing oncofertility care coordination, overcoming the issues in individual regions.
Keywords: cryopreservation, fertility preservation, oocytes, ovary, survivorship
1. INTRODUCTION
Innovation in therapeutic strategies for treating cancer, such as hematopoietic cell transplantation, high‐dose chemotherapy, radiation, and surgery, have led to better survival outcomes in pediatric and young adult (AYA) (aged 15‐39 years) patients. However, the gonadal toxicity and/or gonadal resection that often are associated with these therapeutic strategies can result in iatrogenic infertility. In Japan, there are an estimated 1875 child patients (aged 0‐14 years) with cancer and 21 572 adolescent and AYA patients with cancer annually.1 Based on the 10 year relative survival rates,2 >5500 men and >11 000 women annually could experience the aforementioned reduction in, or loss of, fertility that is associated with being young “cancer survivors.”
Fertility preservation has been a focus in the field of cancer supportive care in the last decade. The objective of fertility preservation is not merely the cryopreservation of oocytes, ovarian tissue, and sperm, but also the maintenance of favorable cancer treatment outcomes, along with fertility for safe gestation and delivery for both the mothers and their infant. On the other hand, “oncofertility” refers not only to the preservation of fertility itself, but also to the general medical care that supports patients with cancer in making their own decisions regarding future pregnancies and births, as they undergo cancer treatment. “The aim of oncofertility is not exclusively fertility preservation for cancer patients, but the reexamination of the meaning of having children, including the option of a life without children” (Yoshimura Y, 2017, personal communication).
As a facet of fertility preservation, unfertilized oocytes can be cryopreserved for women as early as puberty. However, the cryopreservation of oocytes almost always requires ovarian stimulation by follicle‐stimulating hormone (FSH) preparations, which can delay cancer treatment. Additionally, this method yields no more than 20 oocytes, which might not be sufficient to achieve pregnancy.
In contrast, with the cryopreservation of ovarian tissue, samples can be harvested immediately by using minimally invasive laparoscopic surgery, even in prepubertal girls. In addition, because the ovarian tissue cortex contains thousands of primordial follicles, it is possible to obtain a far greater number of oocytes and a much higher pregnancy rate, even when accounting for the damage that is caused by cryopreservation, thawing, and transplantation. In one recent study, immature oocytes were harvested from excised ovarian tissue and cryopreserved following in vitro maturation (IVM).3 In another study, oogonial stem cells were isolated from cryopreserved ovarian tissue.4, 5 A successful live birth also has been achieved by the intracytoplasmic sperm injection of immature oocytes that had been harvested from ovarian tissue that had been resected from a patient with advanced ovarian cancer and then transferring the resulting embryos into her uterus, which was preserved following chemotherapy.6
As described above, there are several methods of fertility preservation for young female patients with cancer, each of which has its own advantages and disadvantages (Table 1); the best outcomes might arise from a combination of multiple methods, determined on a case‐by‐case basis. It also has been reported that an equivalent quantity and quality of oocytes have been obtained and cryopreserved through ovarian stimulation, even after harvesting and cryopreserving half of the unilateral ovarian tissue.7
Table 1.
Fertility preservation for female patients with cancer
| Variable | Embryo | Oocyte | Ovarian tissuea |
|---|---|---|---|
| Main indications | Leukemia, breast cancer, lymphoma, gastrointestinal cancer, gynecologic cancer, malignant melanoma, germ cell tumor, brain tumor, sarcoma etc. | Leukemia, breast cancer, lymphoma, gastrointestinal cancer, gynecologic cancer, malignant melanoma, germ cell tumor, brain tumor, sarcoma etc. | Breast cancer, lymphoma etc. (when considering auto‐transplantation) |
| Target age | 16‐45 years | 14‐40 years | 0‐40 years (can be performed for children) |
| Marital status | Married | Single/married | Single/married |
| Treatment duration | 2‐8 wks | 2‐8 wks | 1‐2 wks |
| Cryopreservation method | Vitrification | Vitrification | Slow freezing or vitrification |
| Post‐thawing survival rate | ≥95‐99% | ≥90% | ≥90% |
| Cost in Japan (USD) | 3000‐4500 | 2000‐3500 | 5500‐7000 (+5500‐7000 for transplantation) |
| Number of births | ≥40 000/y in Japan alone | ≥6000 worldwide | ≥100 worldwide (research stage) |
| Pregnancy rate | Pregnancy rate of 30%‐40% per embryo | Pregnancy rate of 4.5%‐12% per oocyte | Pregnancy rate of 20%‐30% per transplantationa |
Potential disease recurrence due to transplantation.
One conventional fertility preservation method is “ovarian protection” therapy using gonadotropin‐releasing hormone (GnRH) agonists to preserve the ovarian function against chemotherapy, which is a simpler method than the cryopreservation of oocytes or ovarian tissue. Using this method, a 2015 study reported a significant improvement in the ovarian function and fertility of patients with breast cancer8; however, these findings have been inconsistent. A more recent, prospective, randomized trial reported a lack of protective effect from GnRH agonists for the ovarian reserve or fertility of patients with lymphoma.9 Further studies are necessary, including a strict assessment of the ovarian reserve by measuring serum anti‐Müllerian hormone (AMH) and longer term follow‐ups of patients.10
2. CURRENT STATUS OF FERTILITY PRESERVATION IN JAPAN AND OTHER COUNTRIES
Fertility preservation guidelines first were published by the American Society of Clinical Oncology in 2006. The guidelines state that all healthcare providers should heed the possibility of cancer treatment‐induced infertility and refer patients who request fertility preservation to reproductive specialists as soon as possible, prior to initiating treatment.11 Outside of Japan, oocytes are cryopreserved for the sake of oocyte banking. This procedure has yielded an estimated several thousand successful live births.12 The FertiPROTEKT oncofertility network, which comprises more than 100 centers in Germany, Switzerland, and Austria, has published guidelines regarding indications for the cryopreservation of oocytes and ovarian tissue.13 As of 2015, ovarian tissue cryopreservation has been conducted for ~2400 patients, which is several times more than those who underwent oocyte and embryo cryopreservation.14 The American Society of Reproductive Medicine (ASRM) has stated in its guidelines that oocyte cryopreservation should no longer be confined to clinical research, but instead should be considered as an effective method of oncofertility, based on appropriate patient counseling.15 However, the ASRM also expressed the opinion that the cryopreservation and transplantation of ovarian tissue should be pursued carefully as clinical research.16 The British National Institute for Health and Clinical Excellence also has published a guideline that holds oocyte cryopreservation to be a useful reproductive technology.17
Japan, in contrast, has pioneered and spread vitrification, a method whereby coincidental oocyte cryopreservation has been performed, which has resulted in live births for some patients with infertility who could not obtain their partners’ sperm at the time of oocyte retrieval. The 21 centers that belong to the Japan Association of Private Assisted Reproductive Technology Clinics and Laboratories have been engaged in oocyte cryopreservation for unmarried women with diseases, such as leukemia, since 2007. Two successful pregnancies were reported in March 2012 as a result of 151 oocyte pick‐up cycles in 82 patients.18 The first fertility preservation guideline in Japan was published in 2014 for patients with breast cancer in response to the above‐mentioned domestic and international trends. This guideline strongly recommends communication between oncologists and reproductive specialists regarding fertility preservation.19 Both the Japan Society for Reproductive Medicine20 and the Japan Society of Obstetrics and Gynecology (JSOG)21 have published statements regarding oocyte and ovarian tissue cryopreservation, initiating the standardization and spread of the technology. In 2016, a revised JSOG statement referred to the cryopreservation of embryos based on medical indications, in addition to the cryopreservation of oocytes and ovarian tissue.22 The JSOG mentioned fertility preservation in its clinical practice guidelines for the first time in 2017. In addition, the Japan Society of Clinical Oncology issued fertility preservation guidelines for children, adolescent, and AYA patients with female reproductive, mammary, urinary, pediatric, hematologic, bone or soft tissue, cerebral, and gastrointestinal malignancies.
3. RECENT ISSUES IN ASSISTED REPRODUCTIVE TECHNOLOGY FOR FERTILITY PRESERVATION
3.1. Suitable timing of oocyte retrieval for patients with cancer
Oocyte retrieval preferably is performed prior to the initiation of chemotherapy; however, because retrieval cannot be performed until at least 10‐14 days after ovarian stimulation has begun, some patients with cancer cannot wait for oocyte retrieval to begin treatment. In the case of adjuvant chemotherapy for breast cancer, for which there is more abundant evidence than for any other type of cancer, most studies consider 12 weeks to be the maximum tolerable delay before starting treatment following fertility preservation.23 For neoadjuvant chemotherapy, although delays in the initiation of treatment are not generally tolerated, there has not been an observed decrease in the 5 year survival rate, as long as the treatment is initiated within 6 weeks after diagnosis.24 Acute leukemia, in contrast, must be treated immediately, making it impossible to take the time necessary for oocyte retrieval. Therefore, the suitability of oocyte retrieval only can be examined after several courses of chemotherapy have been performed. Clear evidence is lacking for other types of cancer; therefore, the opportunity for oocyte retrieval is currently determined based on discussions between the oncologists treating the primary malignancy and reproductive specialists.
With regard to the timing of oocyte retrieval following chemotherapy, a study of in vitro fertilization using mice that had been injected with cyclophosphamide 6 weeks earlier found that both the fertilization and embryonic developmental rates were significantly reduced, compared to the controls. Furthermore, the percentage of aneuploid embryos was significantly increased, compared to the control group.25 Although the children who are born to the cancer survivors generally are not considered to have an increased incidence of congenital anomalies, some studies have reported increased incidences of spontaneous abortions, preterm births, and low‐birthweight infants. Although there is little evidence to suggest that oocyte retrieval, immediately following chemotherapy completion, affects the outcomes of the resulting infants, sufficient patient education, strict patient management, and careful follow‐up are necessary.
3.2. Random‐start protocol
In typical controlled ovarian stimulation, the stimulation is begun shortly after menstruation; therefore, based on the woman's menstrual cycle, it might be necessary to wait 2‐6 weeks before administering fertility drugs. However, an increasing number of young patients with cancer, for whom a certain level of ovarian reserve is expected and fresh embryo transfer in the oocyte retrieval cycle is not necessary, are undergoing “random‐start protocols.” In these protocols, ovarian stimulation is begun, regardless of the patient's menstrual cycle. Although there was an initial worry that the progesterone that is secreted in the luteal phase would negatively affect the quality of the oocytes, many recent studies have found this not to be the case.26
In random‐start protocols, ovarian stimulation is begun in either the late follicular or the luteal phase. The drugs that are used to inhibit the spontaneous luteinizing hormone surge include not only the conventional GnRH antagonists, but also medroxyprogesterone acetate.27 In a recent meta‐analysis of a total of 338 women who underwent ovarian stimulation (251 of whom did so for fertility preservation) that began in the luteal phase,28 the duration and dosage of the FSH preparations were significantly higher than in a control group of women, in whom ovarian stimulation was initiated in the early follicular phase. However, there were no significant differences in the number of retrieved oocytes, cryopreserved embryos, or peak estradiol levels. Similar results were found in a subanalysis that was limited to women undergoing ovarian stimulation for fertility preservation. In another analysis of 684 women that was reported by FertiPROTEKT,29 the duration of ovarian stimulation and the total dose of the FSH preparations were significantly greater in the group of women who began ovarian stimulation in the luteal phase (after day 14) than in either of the two other groups (initiation in the early and late follicular phases), while the number of retrieved oocytes was significantly higher in the two random‐start groups (initiation in the late follicular and luteal phases). These findings suggest that in random‐start protocols, ovarian stimulation lasts 1‐2 days longer, resulting in an increase in the dosage of the fertility drugs; however, the number of retrieved oocytes is similar to the number that is retrieved with the conventional protocol. For the ovarian stimulation of infertile women that was initiated in the luteal phase, the pregnancy rate that was achieved with cryopreserved embryos was reported to be similar to the rates for conventional ovarian stimulation28; however, there are currently few studies featuring a comparison of pregnancy rates between the random‐start and conventional stimulation protocols and no study of the pregnancy rates in women who are undergoing ovarian stimulation for fertility preservation.
There was no significant difference in the duration from diagnosis to the initiation of chemotherapy between 58 patients with breast cancer who underwent ovarian stimulation and oocyte retrieval in a random‐start protocol prior to the initiation of neoadjuvant chemotherapy and 29 patients with breast cancer who did not undergo oocyte retrieval. Both groups of women began chemotherapy within 6 weeks of their breast cancer diagnosis.30 The women who underwent oocyte retrieval were referred to a reproductive specialist significantly earlier (~9 days) after their breast cancer diagnosis than the women who did not undergo oocyte retrieval, a finding which reconfirms the importance of early referral to reproductive specialists. Also, while the FertiPROTEKT guideline13 does not recommend preoperative ovarian stimulation for patients with estrogen receptor‐positive (ER‐positive) breast cancer, 37 ER‐positive patients in this study underwent ovarian stimulation, which yielded an important finding: the use of letrozole suppressed the peak estradiol levels at a mean of 889 ± 655 pg/mL. Long‐term prognosis data are not yet available for this patient cohort.
3.3. Optimal ovarian stimulation
The achievement of favorable outcomes in assisted reproduction typically requires multiple, good‐quality embryos. Multiple oocytes are needed to obtain good‐quality embryos because of their poor progression from fertilization to embryo development in vitro. In a randomized, controlled (RCT) study that compared the outcomes between cryopreserved oocytes and fresh oocytes, the fertilization and pregnancy rates were similar, with the clinical pregnancy rate per thawed oocyte ranging from 4.5% to 12%.15 However, the majority of the oocytes examined in this RCT trial were derived from young oocyte donors and infertile women with a favorable ovarian reserve; therefore, further studies are necessary to determine whether the above result can be generalized to all age groups, all infertility treatment centers, and oncofertility. In a multicenter study of 1468 women who underwent oocyte vitrification because of age or for medical reasons other than cancer, the women who underwent oocyte cryopreservation at ≤35 years of age demonstrated an increase in the cumulative live birth rates following oocyte thawing as more oocytes were used, reaching a peak rate of 85.2% with 15 oocytes.12 This result confirmed that fertility preservation requires ~10‐15 oocytes. In contrast, among the women who underwent oocyte cryopreservation at ≥36 years of age, the cumulative birth rate plateaued at 35.6% with 11 oocytes. This result indicates that the age at oocyte retrieval affects the fertility preservation outcome.12
As stated earlier, oncofertility poses greater time constraints than infertility; therefore, ovarian stimulation is necessary to obtain as many oocytes as possible in a short time. However, the bleeding, infection, and other complications that result from ovarian hyperstimulation syndrome (OHSS) and the oocyte retrieval that is associated with ovarian stimulation can delay the treatment of the primary disease. Controlled ovarian stimulation using GnRH antagonists is less likely to result in OHSS than in those using GnRH agonists; therefore, controlled ovarian stimulation using GnRH antagonists is recommended for fertility preservation. The FertiPROTEKT guideline13 also recommends ovarian stimulation with GnRH antagonists, rather than GnRH agonists, because of the lower risk of OHSS. In Japan, however, there is an increasing use of mild ovarian stimulation with clomiphene,31 including in oncofertility. A study that was conducted with infertile patients32 found that controlled ovarian stimulation using GnRH agonists yielded more cycles in which embryos could be obtained for cryopreservation, compared to mild ovarian stimulation, even though the two groups demonstrated equal pregnancy rates. Although the minimal use of ovarian stimulation is particularly important for patient safety in oncofertility, further studies are necessary in order to determine whether ovarian stimulation methods affect clinical outcomes, such as pregnancy rates.
3.4. Aromatase inhibitors for estrogen‐dependent malignancies
In assisted reproductive technology (ART), controlled ovarian stimulation can elevate estradiol in the blood to non‐physiological levels, thereby possibly accelerating tumor development in ER‐positive breast cancer and other estrogen‐dependent malignancies. It has been reported that this negative effect can be inhibited by controlled ovarian stimulation that combines gonadotropins with letrozole, an aromatase inhibitor (AI). Originally developed as a therapeutic agent for ER‐positive breast cancer in postmenopausal women, AIs inhibit the activity of aromatase, which synthesizes estrogen, increases the level of FSH in the blood, promotes follicular development, and reduces the level of estradiol in the blood. A 2001 study reported on the clinical application of AIs for clomiphene‐resistant anovulation.33 A RCT also reported that in women with polycystic ovary syndrome, ovarian stimulation with AIs yielded a higher live birth rate than did stimulation with clomiphene.34 The data that were reported at the ASRM's annual meeting in 2005 suggested that letrozole is teratogenic; however, in a RCT that used letrozole for 900 women with unexplained fertility, there was no increase in the rate of major anomalies.35 A study of infertile Japanese women that compared the data between 3136 natural cycles and 792 letrozole‐induced cycles, both of which were associated with fresh, single‐embryo transfer and resulted in a clinical pregnancy, revealed that the risk of miscarriage was, in fact, significantly lower in the letrozole group, while the two groups were equal in terms of major congenital anomalies and the risk for any specific organ system.36
Few studies have compared the treatment outcomes between controlled ovarian stimulation combined with letrozole and conventional controlled ovarian stimulation. In one such study that compared 142 cycles, in which letrozole was combined with a GnRH antagonist protocol, to 97 cycles conducted with a typical GnRH antagonist protocol, the number of oocytes that was retrieved was significantly lower in the former group despite equal doses of FSH preparations.37 However, in another study in which the embryos were cryopreserved from controlled ovarian stimulation combined with letrozole for 131 patients with breast cancer, when 81 thawed embryos were transferred in 40 cycles for 33 patients, 25 babies (31.3% per embryo) were born from 18 cycles (45.0% per embryo transfer).38 The live birth rate per embryo transfer was similar among the infertile women in the USA who were of a similar age (38.2%, 35‐37 years) at the time of the oocyte retrieval. In addition, there was no anomaly among the 25 babies, nor any developmental abnormality detected during a mean follow‐up period of 40.4 months. In a study of breast cancer treatment outcomes at a single center, in which 120 patients with breast cancer who underwent fertility preservation (oocyte or embryo cryopreservation) by ovarian stimulation that was supplemented with letrozole were compared with 227 patients with breast cancer who did not undergo fertility preservation, the two groups did not demonstrate significant differences in the breast cancer recurrence rate or patient survival outcomes.39
Little is known about the relationship between progesterone and breast cancer development. Progesterone, after being converted into androgens by 17 alpha hydroxylase and C17,20‐lyase, then is converted by aromatase into estrogens.40 Therefore, AIs, such as letrozole, might trigger progesterone accumulation. In ovarian stimulation with letrozole, letrozole administration often is continued following oocyte retrieval in order to avoid an elevation in the estradiol levels. Additionally, the administration of GnRH antagonists following oocyte retrieval has been recommended to avoid elevations in the estradiol and progesterone levels.41 Further study is considered to be necessary in order to elucidate the optimal stimulation protocol that minimizes the risk of breast cancer development.
3.5. Other assisted reproductive technologies, including in vitro maturation
As stated earlier, ovarian stimulation is preferable in cases of fertility preservation. However, when there is very little available time, natural‐cycle oocyte retrieval can be performed rather than ovarian stimulation in order to preserve at least some level of future fertility. Through the IVM of immature oocytes, aspirated from small follicles, it is possible to obtain multiple mature oocytes without conducting ovarian stimulation, thus making IVM a potentially effective strategy. In a recent study with 248 patients with breast cancer who were scheduled to undergo chemotherapy, who had a relatively favorable ovarian reserve (a total of ≥11 antral follicles in both ovaries), an oocyte retrieval was performed 36 hours after the administration of human chorionic gonadotropin preparation in the natural cycles (in the follicular phase for 127 patients and in the luteal phase for 121 patients). After 48 hours of IVM, despite the difference in the menstrual cycle phases, the two groups had equal numbers of oocytes retrieved, oocyte maturation rates, and numbers of cryopreserved mature oocytes.42
There are few studies on the oocyte retrieval strategies that are suitable for fertility preservation in patients with a poor ovarian reserve. One such study proposed a two‐stage ovarian stimulation method that consisted of oocyte retrieval via mild ovarian stimulation using clomiphene or letrozole, followed by the initiation of controlled ovarian stimulation in the subsequent luteal phase.43 Another study reported that two consecutive cycles of ovarian stimulation yielded significantly higher numbers of oocytes and embryos than a single‐cycle ovarian stimulation with a similar recurrence rate.44 However, there are currently few studies on the cancer treatment outcomes and pregnancy rates among those patients who have undergone ART for oncofertility. Therefore, it is essential to carefully discuss the indications and guidelines with patients, to accumulate and follow up with patients, and to conduct further analyses, not only of the cancer treatment outcomes, but also of the pregnancy outcomes.
3.6. Prenatal and neonatal risks from oocyte and embryo cryopreservation
In a study of >900 neonates who were born from oocytes that had been cryopreserved by slow freezing, the rate of congenital anomalies was similar to that of the general population.45 In a comparison of 1027 neonates from 804 pregnancies using vitrified oocytes to 1224 neonates from 996 pregnancies using fresh oocytes, there were no significant differences in obstetric complications (preeclampsia, preterm birth etc.), gestational age at delivery, birthweight, Apgar scores, birth defects, admission to a neonatal intensive care unit, perinatal mortality, or puerperal complications.46
However, even when techniques, such as a single‐embryo transfer, are used to reduce the incidence of multiple births, the incidence of a low birthweight in singletons who are born through ART is reported to be higher than among singletons who are born through spontaneous conception.47, 48 Also, the birthweight is reported to be higher in cryopreserved embryo transfer pregnancies than in fresh embryo transfer pregnancies.47, 49 In a large‐scale Japanese analysis, the birthweights of neonates who were born from cryopreserved embryos were significantly larger than the typical Japanese birthweights (as defined by statistics from the Japanese Ministry of Health, Labour and Welfare; MHLW).50 In another large‐scale Japanese analysis, preterm births, low‐birthweight infants, and small‐for‐gestational age infants were significantly less frequent with cryopreserved embryos than with fresh embryos.51 The birthweight was reported to be significantly higher in cryopreserved embryos who were transferred under estrogen/progesterone supplementation than in cryopreserved embryos who were transferred from natural cycles,50 a finding that suggests the involvement of the intrauterine environment early in pregnancy. However, a Japanese study reported that cryopreserved embryo transfer is associated with a higher incidence of placenta accreta and preeclampsia,51 a finding that highlights the need for continuous examination of the effects of oocyte cryopreservation and embryo cryopreservation on both the mother and the infant.
Vitrification is performed primarily by directly immersing oocytes and embryos in liquid nitrogen in an open device. However, this method involves a theoretical possibility of infection by pathogens via the liquid nitrogen. Although there has been no reported case of infection to date,52, 53 there is another method of vitrification using a closed device, which prohibits the direct contact between the embryos and the liquid nitrogen. Although there is considered to be no difference in the pregnancy rates or other treatment outcomes between the open and closed devices, only one single‐center RCT has been performed to date.54 Currently, in Japan, many centers continue to use open devices.
4. OVARIAN TISSUE CRYOPRESERVATION: PRACTICE AND PROBLEMS
4.1. Slow freezing and vitrification
In slow freezing, a controlled‐rate freezer is used to freeze tissue gradually at a rate of 0.5°C per minute to ~ −35°C. Vitrification, which uses higher concentrations of cryoprotectants than slow freezing, is a cryopreservation method in which the tissue is frozen rapidly by immersion in liquid nitrogen. It has been reported that the survival rate, fertilization rate, and the rate of pregnancies per thawed oocyte all were significantly higher with vitrification than with slow freezing.55 However, since the first‐ever report of the cryopreservation of ovarian tissue in 2004,56 slow freezing has been the predominant method for ovarian tissue cryopreservation, which has been reported to have produced at least 130 successful pregnancies and live births.57 One problem that is posed by slow freezing is that because it requires a controlled‐rate freezer, slow freezing can be performed only at a limited number of medical centers, to which excised ovarian tissue therefore must be transported. Another problem is that the cryopreservation program takes a relatively long time of two‐to‐three hours. Two types of vitrification, the Cryotissue method58 and the Cryosupport method,59 have been reported. Both methods enable cryopreservation at the patient's bedside in the operating room within one hour of tissue harvesting; therefore, these methods have been growing in prevalence, primarily in Asia. A recent meta‐analysis found no significant differences between vitrification and slow freezing in the percentage of intact primordial follicles or in the primordial follicle density, but did find vitrification to be associated with significantly less DNA damage and a significantly higher number of stromal cells.60 Although live births have been achieved by vitrification and the auto‐transplantation of ovarian tissue that has been harvested from patients with primary ovarian insufficiency,61 there has been no report of pregnancies being achieved by vitrification and the auto‐transplantation of ovarian tissue in oncofertility. One study found that the residual concentration of cryoprotectants after thawing was significantly higher with the Cryotissue method than with slow freezing.62 The clinical efficiency of ovarian tissue cryopreservation by vitrification or slow freezing cannot be compared until the pregnancy outcomes for auto‐transplantation by vitrification are known, which probably will take several years.
4.2. Auto‐transplantation
The uses for excised ovarian tissue are outlined in Figure 1. Currently, only auto‐transplantation has been applied to the clinical setting. Following transplantation, the resumption of the follicular development in ovarian tissue and the recovery of ovarian tissue function typically takes 4‐5 months. In orthotopic transplantations, ovarian tissue fragments are transplanted into the residual ovarian tissue or the broad ligament, whereas in heterotopic transplantation, ovarian tissue fragments are transplanted to the subcutaneous site of the abdominal wall or forearm. According to a recent review, >130 live births have been achieved,57, 63 almost all by orthotopic transplantation. One advantage of heterotopic transplantation is that both the transplant surgery and excision, in the event of recurrent malignancy, of the transplant tissue are easier to perform. Another advantage is that heterotopic transplantations can be used in cases in which factors, such as radiation, make orthotopic transplantation difficult. The use of ART for ovarian tissue that is heterotopically transplanted to the anterior abdominal wall has resulted in a live birth.64
Figure 1.
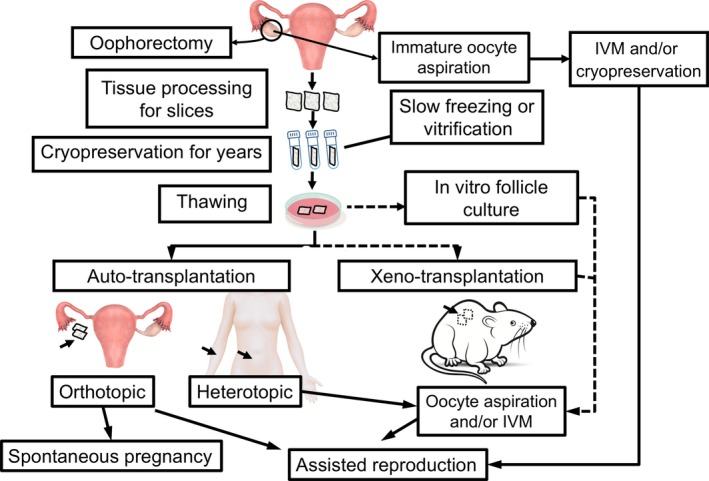
Usages of cryopreserved ovarian tissue. The solid lines represent clinically applied methods that have achieved pregnancies and births, while the dotted lines represent methods that are in the research stage. IVM, in vitro maturation
Multiple studies have reported that the successful live birth rate per transplantation is approximately 30%57, 65, 66; however, these reports have included patients who did not present with ovarian insufficiency prior to transplantation, as well as cases of retransplantation. As recently reported by FertiPROTEKT, single auto‐transplantation for 40 women with ovarian insufficiency yielded a live birth rate of 22.5%.67
The sites and methods for orthotopic transplantation vary according to the medical center. The reported sites include the denuded medulla,68 subcortical tunnels,69 and subcortical pockets70 of the residual ovary. For cases with no residual ovary, the creation of peritoneal windows in both the broad ligament anterior leaf71 and posterior leaf72 have been reported. However, no conclusion has been reached as to which site or method is superior. In light of this, the International Society for Fertility Preservation has created an online registry for ovarian tissue transplantation (http://www.isfpregistry.org) and has begun examining the clinical outcomes.
Even though ovarian tissue cryopreservation might seem to be the only method of fertility preservation in prepubertal female patients with cancer, there are merely two reported cases of auto‐transplantation resulting in pregnancy and a live birth.73, 74 In order to assess the efficacy of ovarian tissue cryopreservation for prepubertal girls, more cases need to be reported.
4.3. Xeno‐transplantation
There have been attempts at xeno‐transplantation of cryopreserved and thawed human ovarian tissue into the ovarian bursa, subcutaneous tissue, muscle, and renal subcapsule of immunodeficient mice. In one study, thawed cryopreserved human ovarian tissue was xeno‐transplanted into the back muscle of severe combined immunodeficient mice and the tissue was stimulated with FSH. The authors then punctured the developed follicles and retrieved metaphase II (MII) oocytes.75 Although xeno‐transplantation does not involve the risk of malignancy recurrence that is associated with auto‐transplantation, it does pose unique problems, including: the transfer of pathogenic substances from the host animal into the oocytes; the quality of the human oocytes as developed in an animal; and various safety and ethical issues. Therefore, for the time being, it might be useful to compare various drugs for increasing the rate of engraftment of the transplanted tissue and to conduct basic analyses of the mechanism of human follicular development.
4.4. In vitro follicular growth systems
In a reported study with two‐step, serum‐free follicle culture, human ovarian tissue that contained primordial follicles was cultured in vitro and grown into preantral follicles, which were then isolated and cultured in the presence of activin A to facilitate their growth into antral follicles.76 The protocol for this method was to culture and grow primordial follicles that had been derived from cryopreserved human ovarian tissue, isolate the oocytes from the resulting antral follicles, and perform IVM in order to obtain mature human MII oocytes. Recently, the research group reported that nine MII oocytes with meiotic spindle formation were obtained by using their culture method; however, all the polar bodies of the MII oocytes were abnormally large and their developmental potential remained unknown.77 Therefore, it is necessary to accumulate more findings that are related to the physical and biochemical factors that contribute to the growth and differentiation of follicles.78
4.5. Oogonial stem cells
It could be considered the “central dogma” of reproductive medicine that primordial germ cells within ovaries continue to dwindle after birth and are not regenerated or replenished. By contrast, a study reported findings in 2004 that suggested follicular renewal in the ovaries of adult mice.79 It also has been reported by multiple studies that similar to Drosophila and teleosts, adult mouse ovaries have a small number of reproductive cells that are capable of proliferation, which are able to produce eggs, and even offspring.80 Finally, in 2012, mitotically active oogonial stem cells (OSCs) were isolated from cryopreserved human adult ovarian tissue.4 When these human OSCs were cultured, they produced large cells that were 35‐50 μm in diameter and these enlarged cells expressed the terminal oocyte markers, such as GDF‐9, zona pellucida glycoproteins, and newborn ovary homeobox, as well as meiosis markers, such as Y‐box protein 2 and synaptonemal complex protein 3. As fluorescence‐activated cell sorting‐based ploidy analysis of the cultured human OSCs detected a cell population that exhibited the haploid status, it was suggested that cryopreserved ovarian tissue could be the source of proliferative OSCs that might differentiate into haploid oocyte‐like cells in vitro.
A number of skeptical reviews and rebuttals have arisen in response to these reports of oogonial stem cells in ovaries.81, 82 Although there has been no scientific consensus, there recently has been a similar report from another research group,5 indicating an acceleration in the research using OSCs in the field of reproductive medicine. The Japanese policy designating the handling of stem cells is that oocytes and sperm[s] that have been produced from stem cells shall not be used for fertilization.83 Nevertheless, amid rising expectations for the results of further research, there is likely to be a need for a specific, wide‐ranging discussion regarding the stage to which such research may be permitted to proceed.
4.6. Follicular loss after transplantation
According to current methodologies, several days are required for sufficient angiogenesis in the transplanted tissues to facilitate the recovery from hypoxia after ovarian tissue transplantation.84 In this process, it is estimated that 25%‐90% of the primordial follicles are lost, probably related to follicle “burnout” that is associated with primordial follicle recruitment following transplantation.85, 86 Consequently, the transplanted ovarian tissue can function anywhere from 2 to 3 months to as long as 5 years. In order to reduce the loss of primordial follicles in transplanted ovarian tissue, methods such as the creation of a peritoneal window 1 week prior to transplantation56 or the incision of the residual ovarian tissue to serve as the transplantation site, have been attempted in order to achieve local angiogenesis.87 However, as stated previously, no conclusion has been reached as to which site or method is superior. Antioxidants, such as vitamin E,87 sphingosine‐1‐phosphate, which possesses anti‐apoptotic effects,88 hormones such as gonadotrophins and GnRH analogs,87 vascular endothelial growth factor,89 basic fibroblast growth factor,90 angiopoietin‐291 and other cytokines with an angiogenic effect, extracellular tissue matrices, such as a human extracellular matrix scaffold,66 and endothelium that continuously expresses follicular activation‐suppressing AMH92 all have been reported to be effective in the reduction of follicular loss in both the xeno‐transplantation experimental system and in clinical practice.
4.7. Residual malignant cells in the transplanted tissue
It has been indicated that the transplanted ovarian tissue could contain malignant cells (minimal residual disease; MRD). Although there is no sufficient evidence, there has been no report of disease recurrence associated with reintroduction; thus, it is highly likely that the auto‐transplantation of ovarian tissue can be performed safely, as long as the type and stage of malignancy are taken into account. According to a recent review,93 Hodgkin's lymphoma, non‐Hodgkin's lymphoma, and breast cancer all were considered to be indications for human ovarian tissue cryopreservation.
When thawing and transplanting cryopreserved ovarian tissue, in addition to providing the patient with sufficient information, it is advisable to first evaluate the presence of MRD by conducting histopathology tests, immunostaining, and the detection of genetic mutations (such as by polymerase chain reaction or next‐generation sequencing) on a portion of the transplant tissue. At present, the most effective method is considered to be the observation of the tissue in xeno‐transplantation for ≥20 weeks.93
Auto‐transplantation has been considered to be best avoided in cases of leukemia; however, because of the anticipation of developments from future research, cryopreservation often is performed for patients with leukemia, as well. The Pediatric Diseases Working Party, which includes specialists from the European Society for Blood and Marrow Transplantation, recently has published a guideline for fertility preservation in pediatric patients who are undergoing hematopoietic stem cell transplantation. The guideline states that although the auto‐transplantation of ovarian tissue in patients with leukemia should be avoided because of the lack of a validated method for detecting MRD, the cryopreservation of ovarian tissue can be considered in light of potential future developments in the technology, such as the aforementioned in vitro follicular growth.94 In fact, an Israeli group recently reported that, following sufficient assessment of MRD, the auto‐transplantation of cryopreserved ovarian tissue that had been harvested after inducing the remission of acute myeloid leukemia resulted in a live birth. Furthermore, at two years since transplantation, there has been no recurrence of leukemia.95
5. JAPANESE ONCOFERTILITY CARE DELIVERY SYSTEM
5.1. Coordination between the cancer hospitals and the assisted reproductive technology hospitals and clinics
As stated earlier, many academic societies in Japan have issued guidelines, recommendations, and statements regarding fertility preservation. However, the viability of these guidelines and recommendations is predicated on a well‐developed oncofertility care delivery system that conducts counseling and fertility preservation. The Phase 3 Basic Plan to Promote Cancer Control, which was approved by the Japanese Cabinet in October 2017, stipulates that the Japanese Government shall construct “… a system which enables patients to be referred to centers specializing in suitable reproductive care”;96 therefore, the standardization of oncofertility care can be considered to be an urgent issue of national policy.
As of 2017, Japan had 432 cancer hospitals97 and 15 pediatric cancer hospitals98 that were designated by the MHLW, in addition to 605 ART hospitals or clinics that were registered with the JSOG.99 Among the hospitals or clinics that were registered with the JSOG that conduct fertility preservation (oocyte and/or ovarian tissue cryopreservation),99 as of December 15, 2017, there were 34 hospitals or clinics who dealt with unfertilized oocytes and ovarian tissues, 54 hospitals or clinics who dealt with only unfertilized oocytes, and one hospital that dealt with only ovarian tissue. There were 107 centers that were both MHLW‐designated cancer hospitals and JSOG‐registered ART hospitals or clinics, only 55 of which also conducted some form of fertility preservation (Figure 2). Similarly, there were nine centers that were both MHLW‐designated pediatric cancer hospitals and JSOG‐registered ART hospitals or clinics, only four of which also conducted some form of fertility preservation (Figure 2). Thus, the majority of cancer hospitals do not conduct ART or fertility preservation, a state of affairs that suggests that the delivery of oncofertility care requires the establishment of a system for referring patients to specialized centers.
Figure 2.
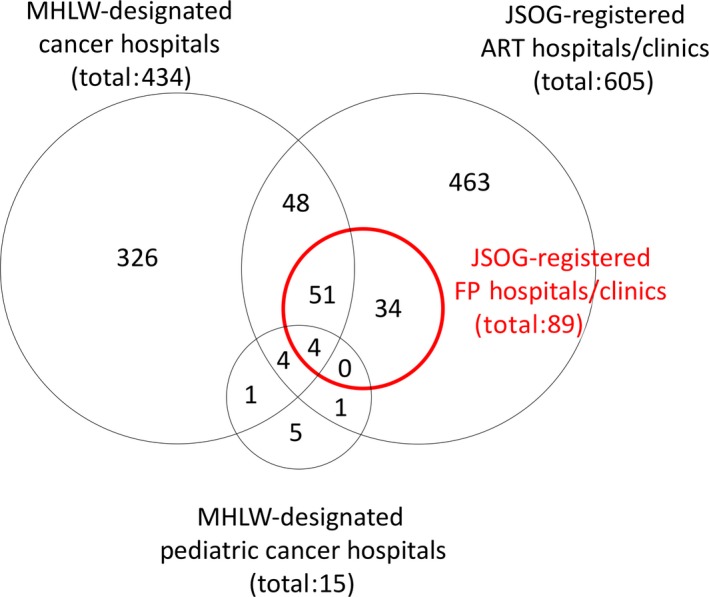
Japanese hospitals and clinics that are engaged in cancer treatment (adult and pediatric), assisted reproductive technology (ART), and fertility preservation (FP). The numbers represent the number of hospitals and clinics belonging to each population. Japan has ~179 000 hospitals and clinics.105 Cancer treatment also is performed at hospitals and clinics other than at Ministry of Health, Labour and Welfare (MHLW)‐designated cancer hospitals, whereas ART and FP (red) are performed only at hospitals and clinics that are registered with the Japan Society of Obstetrics and Gynecology (JSOG)
5.2. Current status of regional oncofertility networks
In order to streamline the coordination between the cancer treatment hospitals and the reproductive care hospitals or clinics in Japan, individual prefectures have been sponsoring the construction of regional oncofertility networks. As of December 2017, oncofertility networks have been established in Gifu,100 Shiga, Saitama, and 18 other prefectures101, 102; however, the activities of these individual networks vary. Specifically, there are three different models: (i) the “flagship hospital model,” in which a university hospital (typically a cancer hospital), of which every prefecture has at least one, is established as the base of the network; (ii) the “reproductive care center‐led model,” which is formed by the reproductive care centers reaching out to the cancer hospitals; and (iii) a mixture model of (i) and (ii). The advantages of the first model are that understanding the state of affairs and enacting policies are relatively easy; however, the policies that are enacted must take into account the characteristics of the region. In major metropolitan areas, such as Tokyo and Osaka, many cancer hospitals and ART hospitals or clinics each coordinate in unique, complex fashions; as a result, it is difficult to establish a comprehensive network or to understand the state of affairs within a given institution. However, the distribution of the JSOG‐registered fertility preservation hospitals or clinics is skewed toward large cities (Figure 3). There are 13 prefectures without JSOG‐registered fertility preservation hospitals or clinics, which indicates that these prefectures’ oncofertility care delivery systems are underdeveloped. Through a questionnaire‐based survey that was conducted of ART hospitals and clinics in regions that were suspected to have underdeveloped systems, it was learned that university hospitals in Iwate, Yamagata, and eight other prefectures conduct ART and oncofertility counseling for cancer patients and have established systems for referring patients to suitable fertility preservation hospitals or clinics outside of the prefecture when necessary. In contrast, university hospitals in the Nara, Saga, and Kagawa prefectures do not conduct ART, but are taking steps to establish counseling and referral systems. However, regions with underdeveloped oncofertility care delivery systems cannot fully develop these systems overnight. Therefore, for the children, adolescent, and AYA patients with cancer, their families, and their attending physicians in these regions, it would be desirable to be able to consult with the secretariat of the Japan Society for Fertility Preservation (JSFP) in a set‐up that is similar to the National Cancer Center Japan's Cancer Treatment and Pregnancy Consultation Service.
Figure 3.
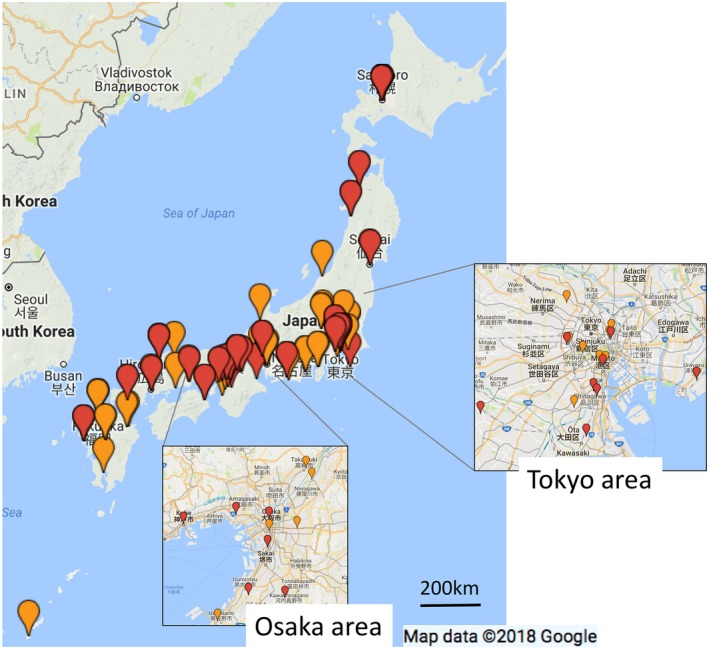
Distribution of the fertility preservation hospitals and clinics in Japan. The red pins represent the hospitals or clinics that cryopreserve both ovarian tissue and unfertilized oocytes, while the orange pins represent the hospitals or clinics that only cryopreserve unfertilized oocytes. The figure demonstrates the concentration of fertility preservation hospitals and clinics in the major metropolitan areas (insets) and the dearth of fertility preservation hospitals and clinics along the Sea of Japan coast and in the Tohoku (north‐eastern) region
5.3. Psychosocial care delivery system based on the coordination among healthcare providers
Providing effective oncofertility care in a smooth fashion requires coordination among the various types of healthcare providers, such as physicians, nurses, psychologists, pharmacists, and social workers. Providing suitable psychosocial care with accurate information and foresight from various specialists enables patients to make complex decisions, even when they are faced with severe psychological stress.103
In the USA, the field of oncofertility includes healthcare providers called “patient navigators,” to whom patients are referred when the oncologist believes the patient should consider fertility preservation. The first task of these patient navigators is to provide information.104 Oncofertility requires close cooperation among healthcare providers. The presence of an oncofertility navigator probably would be effective in reducing the burden on individual providers, making oncofertility care delivery systems easier to maintain, and supporting the regions with underdeveloped oncofertility care networks.
The Japan Society for Reproductive Psychology, in cooperation with the JSFP, conducts seminars for clinical psychologists and trains and certifies psychologists who specialize in oncofertility care. The Japan Society for Reproductive Psychology also conducts seminars for embryologists and certified fertility nurses and trains specialized oncofertility coordinators. It is anticipated that these two different types of “oncofertility navigators” will complement each other to provide psychosocial care. The JSFP, with the support of the Japan Cancer Society, also conducts oncofertility care seminars for social workers in the patient support division of cancer hospitals, at which oncofertility navigator services are anticipated.
6. CONCLUSION
Fertility preservation by the cryopreservation of oocytes and ovarian tissues has come to be viewed as an indispensable technology in oncofertility. However, it must be noted that the findings that are related to oocyte cryopreservation are limited to infertile women with a favorable ovarian reserve at certain hospitals or clinics and that ovarian tissue cryopreservation remains in the clinical research stage. Although no negative effect has been observed on cancer treatment outcomes or in the next generation, it is important to conduct and report long‐term observations with larger numbers of patients. The goal of Japanese oncofertility care is not merely fertility preservation, but the establishment of a nationwide oncofertility care delivery system in which multidisciplinary coordination enables all patients with cancer to receive multidisciplinary care.
DISCLOSURES
Conflict of interest: The authors declare no conflict of interest. Human rights statement and Animal studies: This article does not contain any study with human or animal participants that were performed by any of the authors.
ACKNOWLEDGEMENTS
This work was supported by a Health Science Research Grant from the Ministry of Health, Labor and Welfare, Tokyo, Japan (H27‐Gantaisaku‐Ippan‐005 and H28‐Kodomokosodate to Y.T.). We would like to thank Editage (http://www.editage.jp) for English‐language editing.
Takai Y. Recent advances in oncofertility care worldwide and in Japan. Reprod Med Biol. 2018;17:356–368. 10.1002/rmb2.12214
REFERENCES
- 1. Hori M, Matsuda T, Shibata A, et al. Cancer incidence and incidence rates in Japan in 2009: a study of 32 population‐based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015;45:884‐891. [DOI] [PubMed] [Google Scholar]
- 2. Ito Y, Miyashiro I, Ito H, et al. Long‐term survival and conditional survival of cancer patients in Japan using population‐based cancer registry data. Cancer Sci. 2014;105:1480‐1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang JY, Tulandi T, Holzer H, Tan SL, Chian RC. Combining ovarian tissue cryobanking with retrieval of immature oocytes followed by in vitro maturation and vitrification: an additional strategy of fertility preservation. Fertil Steril. 2008;89:567‐572. [DOI] [PubMed] [Google Scholar]
- 4. White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive‐age women. Nat Med. 2012;18:413‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Silvestris E, Cafforio P, D'Oronzo S, Felici C, Silvestris F, Loverro G. In vitro differentiation of human oocyte‐like cells from oogonial stem cells: single‐cell isolation and molecular characterization. Hum Reprod. 2018;33:464‐473. [DOI] [PubMed] [Google Scholar]
- 6. Prasath EB, Chan ML, Wong WH, et al. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum Reprod. 2014;29:276‐278. [DOI] [PubMed] [Google Scholar]
- 7. Huober‐Zeeb C, Lawrenz B, Popovici RM, et al. Improving fertility preservation in cancer: ovarian tissue cryobanking followed by ovarian stimulation can be efficiently combined. Fertil Steril. 2011;95:342‐344. [DOI] [PubMed] [Google Scholar]
- 8. Moore HC, Unger JM, Phillips KA, et al. Goserelin for ovarian protection during breast‐cancer adjuvant chemotherapy. N Engl J Med. 2015;372:923‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Demeestere I, Brice P, Peccatori FA, et al. No evidence for the benefit of gonadotropin‐releasing hormone agonist in preserving ovarian function and fertility in lymphoma survivors treated with chemotherapy: final long‐term report of a prospective randomized trial. J Clin Oncol. 2016;34:2568‐2574. [DOI] [PubMed] [Google Scholar]
- 10. Roness H, Kalich‐Philosoph L, Meirow D. Prevention of chemotherapy‐induced ovarian damage: possible roles for hormonal and non‐hormonal attenuating agents. Hum Reprod Update. 2014;20:759‐774. [DOI] [PubMed] [Google Scholar]
- 11. Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500‐2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cobo A, Garcia‐Velasco JA, Coello A, Domingo J, Pellicer A, Remohi J. Oocyte vitrification as an efficient option for elective fertility preservation. Fertil Steril. 2016;105:755‐764, e8. [DOI] [PubMed] [Google Scholar]
- 13. von Wolff M, Montag M, Dittrich R, Denschlag D, Nawroth F, Lawrenz B. Fertility preservation in women – a practical guide to preservation techniques and therapeutic strategies in breast cancer, Hodgkin's lymphoma and borderline ovarian tumours by the fertility preservation network FertiPROTEKT. Arch Gynecol Obstet. 2011;284:427‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Germeyer A. SOCIAL FREEZING und die vereinbarkeit von beruf und familie. https://static1.squarespace.com/static/560a328fe4b0e8c4f373857e/t/57206c853c44d81ea19e790b/1461742728020/registerdaten_fertiprotekt_2015.pdf. Accessed May 25, 2018.
- 15. Practice Committees of American Society for Reproductive Medicine, Society for Assisted Reproductive Technology . Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37‐43. [DOI] [PubMed] [Google Scholar]
- 16. Practice Committee of American Society for Reproductive Medicine . Ovarian tissue cryopreservation: a committee opinion. Fertil Steril. 2014;101:1237‐1243. [DOI] [PubMed] [Google Scholar]
- 17. National Institute for Health and Clinical Excellence . Fertility problems: assessment and treatment. http://www.nice.org.uk/guidance/cg156. Accessed May 25, 2018.
- 18. Aono F. The present situation of oocyte cryopreservation. Hematol Frontier. 2012;22:1829‐1838. [Google Scholar]
- 19. Japan Society for Fertility Preservation (ed). Treatment selection and patient support program for supporting and maintaining fertility in breast cancer patients. When breast cancer patients have a desire to bear children in the future, is communication between cancer treatment specialists and reproductive care specialists recommended? In: Handbook on Pregnancy, Birth, and Fertility Treatment for Breast Cancer Patients. Tokyo: Kanehara & Company, Ltd; 2014;14‐16. [Google Scholar]
- 20. Japan Society for Reproductive Medicine . A guideline of oocyte and ovarian tissue cryopreservation for medical indications. http://www.jsrm.or.jp/guideline-statem/guideline_2013_01.pdf. Accessed May 25, 2018.
- 21. Japan Society of Obstetrics and Gynecology . A statement on oocyte and ovarian tissue cryopreservation for medical indications. http://www.jsog.or.jp/ethic/mijyuseiranshi_20140417.html. Accessed May 25, 2018.
- 22. Japan Society of Obstetrics and Gynecology . A statement on oocyte, embryo and ovarian tissue cryopreservation for medical indications. http://www.jsog.or.jp/ethic/mijyuseiranshi_20160625.html. Accessed May 25, 2018.
- 23. Yu KD, Huang S, Zhang JX, Liu GY, Shao ZM. Association between delayed initiation of adjuvant CMF or anthracycline‐based chemotherapy and survival in breast cancer: a systematic review and meta‐analysis. BMC Cancer. 2013;13:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith EC, Ziogas A, Anton‐Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg. 2013;148:516‐523. [DOI] [PubMed] [Google Scholar]
- 25. Barekati Z, Gourabi H, Valojerdi MR, Yazdi PE. Previous maternal chemotherapy by cyclophosphamide (Cp) causes numerical chromosome abnormalities in preimplantation mouse embryos. Reprod Toxicol. 2008;26:278‐281. [DOI] [PubMed] [Google Scholar]
- 26. Kofinas JD, Mehr H, Ganguly N, et al. Is it the egg or the endometrium? Elevated progesterone on day of trigger is not associated with embryo ploidy nor decreased success rates in subsequent embryo transfer cycles. J Assist Reprod Genet. 2016;33:1169‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuang Y, Chen Q, Fu Y, et al. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil Steril. 2015;104:62‐70; e3. [DOI] [PubMed] [Google Scholar]
- 28. Boots CE, Meister M, Cooper AR, Hardi A, Jungheim ES. Ovarian stimulation in the luteal phase: systematic review and meta‐analysis. J Assist Reprod Genet. 2016;33:971‐980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. von Wolff M, Capp E, Jauckus J, Strowitzki T, Germeyer A; FertiPROTEKT Study Group. Timing of ovarian stimulation in patients prior to gonadotoxic therapy: an analysis of 684 stimulations. Eur J Obstet Gynecol Reprod Biol. 2016;199:146‐149. [DOI] [PubMed] [Google Scholar]
- 30. Letourneau JM, Sinha N, Wald K, et al. Random start ovarian stimulation for fertility preservation appears unlikely to delay initiation of neoadjuvant chemotherapy for breast cancer. Hum Reprod. 2017;32:2123‐2129. [DOI] [PubMed] [Google Scholar]
- 31. Kato O, Kawasaki N, Bodri D, et al. Neonatal outcome and birth defects in 6623 singletons born following minimal ovarian stimulation and vitrified versus fresh single embryo transfer. Eur J Obstet Gynecol Reprod Biol. 2012;161:46‐50. [DOI] [PubMed] [Google Scholar]
- 32. Karimzadeh MA, Ahmadi S, Oskouian H, Rahmani E. Comparison of mild stimulation and conventional stimulation in ART outcome. Arch Gynecol Obstet. 2010;281:741‐746. [DOI] [PubMed] [Google Scholar]
- 33. Mitwally MF, Casper RF. Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril. 2001;75:305‐309. [DOI] [PubMed] [Google Scholar]
- 34. Legro RS, Brzyski RG, Diamond MP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Diamond MP, Legro RS, Coutifaris C, et al. Letrozole, gonadotropin, or clomiphene for unexplained infertility. N Engl J Med. 2015;373:1230‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tatsumi T, Jwa SC, Kuwahara A, Irahara M, Kubota T, Saito H. No increased risk of major congenital anomalies or adverse pregnancy or neonatal outcomes following letrozole use in assisted reproductive technology. Hum Reprod. 2017;32:125‐132. [DOI] [PubMed] [Google Scholar]
- 37. Domingo J, Guillen V, Ayllon Y, et al. Ovarian response to controlled ovarian hyperstimulation in cancer patients is diminished even before oncological treatment. Fertil Steril. 2012;97:930‐934. [DOI] [PubMed] [Google Scholar]
- 38. Oktay K, Turan V, Bedoschi G, Pacheco FS, Moy F. Fertility preservation success subsequent to concurrent aromatase inhibitor treatment and ovarian stimulation in women with breast cancer. J Clin Oncol. 2015;33:2424‐2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim J, Turan V, Oktay K. Long‐term safety of letrozole and gonadotropin stimulation for fertility preservation in women with breast cancer. J Clin Endocrinol Metab. 2016;101:1364‐1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stanczyk FZ. Steroid hormones In: Lobo RA, Mishell DRJ, Paulson RJ, Schoupe D, eds. Mishell's Textbook of Infertility, Contraception, and Reproductive Endocrinology. Malden, MA: Blackwell Science; 1997:48‐51. [Google Scholar]
- 41. Goldrat O, Gervy C, Englert Y, Delbaere A, Demeestere I. Progesterone levels in letrozole associated controlled ovarian stimulation for fertility preservation in breast cancer patients. Hum Reprod. 2015;30:2184‐2189. [DOI] [PubMed] [Google Scholar]
- 42. Grynberg M, Poulain M, le Parco S, Sifer C, Fanchin R, Frydman N. Similar in vitro maturation rates of oocytes retrieved during the follicular or luteal phase offer flexible options for urgent fertility preservation in breast cancer patients. Hum Reprod. 2016;31:623‐629. [DOI] [PubMed] [Google Scholar]
- 43. Kuang Y, Chen Q, Hong Q, et al. Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (Shanghai protocol). Reprod Biomed Online. 2014;29:684‐691. [DOI] [PubMed] [Google Scholar]
- 44. Turan V, Bedoschi G, Moy F, Oktay K. Safety and feasibility of performing two consecutive ovarian stimulation cycles with the use of letrozole‐gonadotropin protocol for fertility preservation in breast cancer patients. Fertil Steril. 2013;100:1681‐1685; e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Noyes N, Porcu E, Borini A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod Biomed Online. 2009;18:769‐776. [DOI] [PubMed] [Google Scholar]
- 46. Cobo A, Serra V, Garrido N, Olmo I, Pellicer A, Remohi J. Obstetric and perinatal outcome of babies born from vitrified oocytes. Fertil Steril. 2014;102:1006‐1015; e4. [DOI] [PubMed] [Google Scholar]
- 47. Pinborg A, Wennerholm UB, Romundstad LB, et al. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta‐analysis. Hum Reprod Update. 2013;19:87‐104. [DOI] [PubMed] [Google Scholar]
- 48. Pandey S, Shetty A, Hamilton M, Bhattacharya S, Maheshwari A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta‐analysis. Hum Reprod Update. 2012;18:485‐503. [DOI] [PubMed] [Google Scholar]
- 49. Maheshwari A, Pandey S, Shetty A, Hamilton M, Bhattacharya S. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: a systematic review and meta‐analysis. Fertil Steril. 2012;98:368‐377. e1‐9. [DOI] [PubMed] [Google Scholar]
- 50. Nakashima A, Araki R, Tani H, et al. Implications of assisted reproductive technologies on term singleton birth weight: an analysis of 25,777 children in the national assisted reproduction registry of Japan. Fertil Steril. 2013;99:450‐455. [DOI] [PubMed] [Google Scholar]
- 51. Ishihara O, Araki R, Kuwahara A, Itakura A, Saito H, Adamson GD. Impact of frozen‐thawed single‐blastocyst transfer on maternal and neonatal outcome: an analysis of 277,042 single‐embryo transfer cycles from 2008 to 2010 in Japan. Fertil Steril. 2014;101:128‐133. [DOI] [PubMed] [Google Scholar]
- 52. Molina I, Mari M, Martinez JV, Novella‐Maestre E, Pellicer N, Peman J. Bacterial and fungal contamination risks in human oocyte and embryo cryopreservation: open versus closed vitrification systems. Fertil Steril. 2016;106:127‐132. [DOI] [PubMed] [Google Scholar]
- 53. Cobo A, Bellver J, de los Santos MJ, Remohi J. Viral screening of spent culture media and liquid nitrogen samples of oocytes and embryos from hepatitis B, hepatitis C, and human immunodeficiency virus chronically infected women undergoing in vitro fertilization cycles. Fertil Steril. 2012;97:74‐78. [DOI] [PubMed] [Google Scholar]
- 54. Papatheodorou A, Vanderzwalmen P, Panagiotidis Y, et al. Open versus closed oocyte vitrification system: a prospective randomized sibling‐oocyte study. Reprod Biomed Online. 2013;26:595‐602. [DOI] [PubMed] [Google Scholar]
- 55. Smith GD, Serafini PC, Fioravanti J, et al. Prospective randomized comparison of human oocyte cryopreservation with slow‐rate freezing or vitrification. Fertil Steril. 2010;94:2088‐2989. [DOI] [PubMed] [Google Scholar]
- 56. Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405‐1410. [DOI] [PubMed] [Google Scholar]
- 57. Donnez J, Dolmans MM. Fertility preservation in women. N Engl J Med. 2017;377:1657‐1665. [DOI] [PubMed] [Google Scholar]
- 58. Kagawa N, Silber S, Kuwayama M. Successful vitrification of bovine and human ovarian tissue. Reprod Biomed Online. 2009;18:568‐577. [DOI] [PubMed] [Google Scholar]
- 59. Suzuki N, Hashimoto S, Igarashi S, et al. Assessment of long‐term function of heterotopic transplants of vitrified ovarian tissue in cynomolgus monkeys. Hum Reprod. 2012;27:2420‐2429. [DOI] [PubMed] [Google Scholar]
- 60. Shi Q, Xie Y, Wang Y, Li S. Vitrification versus slow freezing for human ovarian tissue cryopreservation: a systematic review and meta‐anlaysis. Sci Rep. 2017;7:8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kawamura K, Cheng Y, Suzuki N, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 2013;110:17474‐17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nakamura Y, Obata R, Okuyama N, Aono N, Hashimoto T, Kyono K. Residual ethylene glycol and dimethyl sulphoxide concentration in human ovarian tissue during warming/thawing steps following cryopreservation. Reprod Biomed Online. 2017;35:311‐313. [DOI] [PubMed] [Google Scholar]
- 63. Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P, Andersen CY. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen‐thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet. 2017;34:325‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stern CJ, Gook D, Hale LG, et al. Delivery of twins following heterotopic grafting of frozen‐thawed ovarian tissue. Hum Reprod. 2014;29:1828. [DOI] [PubMed] [Google Scholar]
- 65. Meirow D, Ra'anani H, Shapira M, et al. Transplantations of frozen‐thawed ovarian tissue demonstrate high reproductive performance and the need to revise restrictive criteria. Fertil Steril. 2016;106:467‐474. [DOI] [PubMed] [Google Scholar]
- 66. Oktay K, Bedoschi G, Pacheco F, Turan V, Emirdar V. First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozen‐banked ovarian tissue with a human extracellular matrix scaffold using robot‐assisted minimally invasive surgery. Am J Obstet Gynecol. 2016;214(e1–94):e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Van der Ven H, Liebenthron J, Beckmann M, et al. Ninety‐five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Hum Reprod. 2016;31:2031‐2041. [DOI] [PubMed] [Google Scholar]
- 68. Silber S. Ovarian tissue cryopreservation and transplantation: scientific implications. J Assist Reprod Genet. 2016;33:1595‐1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Meirow D, Levron J, Eldar‐Geva T, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318‐321. [DOI] [PubMed] [Google Scholar]
- 70. Andersen CY, Rosendahl M, Byskov AG, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23:2266‐2272. [DOI] [PubMed] [Google Scholar]
- 71. Donnez J, Jadoul P, Pirard C, et al. Live birth after transplantation of frozen‐thawed ovarian tissue after bilateral oophorectomy for benign disease. Fertil Steril. 2012;98:720‐725. [DOI] [PubMed] [Google Scholar]
- 72. Dittrich R, Lotz L, Keck G, et al. Live birth after ovarian tissue autotransplantation following overnight transportation before cryopreservation. Fertil Steril. 2012;97:387‐390. [DOI] [PubMed] [Google Scholar]
- 73. Demeestere I, Simon P, Dedeken L, et al. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum Reprod. 2015;30:2107‐2109. [DOI] [PubMed] [Google Scholar]
- 74. Walsh F. Woman has baby using ovary frozen in childhood. http://www.bbc.com/news/health-38312995. Accessed May 25, 2018.
- 75. Soleimani R, Heytens E, Van den Broecke R, et al. Xenotransplantation of cryopreserved human ovarian tissue into murine back muscle. Hum Reprod. 2010;25:1458‐1470. [DOI] [PubMed] [Google Scholar]
- 76. Telfer EE, McLaughlin M. In vitro development of ovarian follicles. Semin Reprod Med. 2011;29:15‐23. [DOI] [PubMed] [Google Scholar]
- 77. McLaughlin M, Albertini DF, Wallace WHB, Anderson RA, Telfer EE. Metaphase II oocytes from human unilaminar follicles grown in a multi‐step culture system. Mol Hum Reprod. 2018;24:135‐142. [DOI] [PubMed] [Google Scholar]
- 78. De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384:1302‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145‐150. [DOI] [PubMed] [Google Scholar]
- 80. Zou K, Yuan Z, Yang Z, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11:631‐636. [DOI] [PubMed] [Google Scholar]
- 81. Zhang H, Panula S, Petropoulos S, et al. Adult human and mouse ovaries lack DDX4‐expressing functional oogonial stem cells. Nat Med. 2015;21:1116‐1118. [DOI] [PubMed] [Google Scholar]
- 82. Zhang H, Zheng W, Shen Y, Adhikari D, Ueno H, Liu K. Experimental evidence showing that no mitotically active female germline progenitors exist in postnatal mouse ovaries. Proc Natl Acad Sci U S A. 2012;109:12580‐12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ministry of Education Culture Sports Science and Technology . Guidelines on the Research on Producing Germ Cells from Human iPS Cells or Human Tissue Stem Cells. 2010; http://www.lifescience.mext.go.jp/files/pdf/n1567_02r2.pdf. Accessed May 25, 2018.
- 84. Van Eyck AS, Jordan BF, Gallez B, Heilier JF, Van Langendonckt A, Donnez J. Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil Steril. 2009;92:374‐381. [DOI] [PubMed] [Google Scholar]
- 85. Ayuandari S, Winkler‐Crepaz K, Paulitsch M, et al. Follicular growth after xenotransplantation of cryopreserved/thawed human ovarian tissue in SCID mice: dynamics and molecular aspects. J Assist Reprod Genet. 2016;33:1585‐1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gavish Z, Spector I, Peer G, et al. Follicle activation is a significant and immediate cause of follicle loss after ovarian tissue transplantation. J Assist Reprod Genet. 2017;35:61‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod Update. 2009;15:649‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Soleimani R, Heytens E, Oktay K. Enhancement of neoangiogenesis and follicle survival by sphingosine‐1‐phosphate in human ovarian tissue xenotransplants. PLoS ONE. 2011;6:e19475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Shikanov A, Zhang Z, Xu M, et al. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng Part A. 2011;17:3095‐3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kang BJ, Wang Y, Zhang L, Xiao Z, Li SW. bFGF and VEGF improve the quality of vitrified‐thawed human ovarian tissues after xenotransplantation to SCID mice. J Assist Reprod Genet. 2016;33:281‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kong HS, Lee J, Youm HW, et al. Effect of treatment with angiopoietin‐2 and vascular endothelial growth factor on the quality of xenografted bovine ovarian tissue in mice. PLoS ONE. 2017;12:e0184546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Man L, Park L, Bodine R, et al. Engineered endothelium provides angiogenic and paracrine stimulus to grafted human ovarian tissue. Sci Rep. 2017;7:8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet. 2013;30:11‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Balduzzi A, Dalle JH, Jahnukainen K, et al. Fertility preservation issues in pediatric hematopoietic stem cell transplantation: practical approaches from the consensus of the Pediatric Diseases Working Party of the EBMT and the International BFM Study Group. Bone Marrow Transplant. 2017;52:1406‐1415. [DOI] [PubMed] [Google Scholar]
- 95. Shapira M, Raanani H, Barshack I, et al. First delivery in a leukemia survivor after transplantation of cryopreserved ovarian tissue, evaluated for leukemia cells contamination. Fertil Steril. 2017;109:48‐53. [DOI] [PubMed] [Google Scholar]
- 96. Ministry of Health Labour and Welfare . The Phase 3 Basic Plan to Promote Cancer Control [Japanese]. 2017; http://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000196973.pdf. Accessed May 25, 2018.
- 97. Ministry of Health, Labour and Welfare . MHLW‐designated Cancer Core Hospitals (as of 2017/4/1). http://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000162065.pdf
- 98. Ministry of Health, Labour and Welfare . MHLW‐designated Pediatric Cancer Hospitals (as of 2017/1/23). http://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000155766.pdf
- 99. Ethics Committee of Japan Society of Obstetrics and Gynecology . JSOG‐registered facilities (as of 2017/12/1). http://www.jsog.or.jp/public/shisetu_number/
- 100. Furui T, Takenaka M, Makino H, Terazawa K, Yamamoto A, Morishige K‐I. An evaluation of the Gifu Model in a trial for a new regional oncofertility network in Japan, focusing on its necessity and effects. Reprod Med Biol. 2016;15:107‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Furui T, Suzuki N, Morishige K, et al.Regional oncofertility network. http://www.j-sfp.org/aya/tiikirenkei/tiikirenkei.html. Accessed December 31, 2017.
- 102. Suzuki N. Oncofertility in Japan: advances in research and the roles of oncofertility consortia. Future Oncol. 2016;12:2307‐2311. [DOI] [PubMed] [Google Scholar]
- 103. Ito Y, Shiraishi E, Kato A, et al. The utility of decision trees in oncofertility care in Japan. J Adolesc Young Adult Oncol. 2017;6:186‐189. [DOI] [PubMed] [Google Scholar]
- 104. Scott‐Trainer J. The role of a patient navigator in fertility preservation. Cancer Treat Res. 2010;156:469‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ministry of Health, Labour and Welfare . Summary of static/dynamic surveys of medical institutions (Jan 2017). http://www.mhlw.go.jp/toukei/saikin/hw/iryosd/m17/dl/is1701_01.pdf. Accessed May 25, 2018.


