Figure 1.
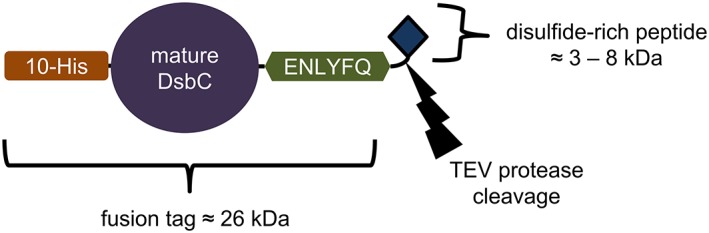
The expression construct created to purify the disulfide‐rich peptides in this study. Peptides were purified via immobilized metal affinity chromatography using the deca‐histidine tag, which is genetically fused to the E. coli disulfide bond isomerase DsbC; the tag is removed from the peptide using TEV protease, which cleaves at the C‐terminal end of the amino acid sequence “ENLYFQ.” The bacterial expression vector for this fusion construct is pCDB364.
