Abstract
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is an uncommon drug hypersensitivity reaction caused by a wide variety of agents. It has a characteristic latent period between 2 and 8 weeks from the onset of drug ingestion followed by a slow resolution with the potential for relapse. Despite being a potentially fatal disease, little is understood about its variable clinical presentation and why it can present long after removal of the offending drug. Visceral organ involvement typically occurs, but rarely results in clinically manifested cardiac injury. In its most aggressive form, acute necrotizing eosinophilic myocarditis (ANEM) can present with DRESS. We present an unusual case of DRESS syndrome due to lamotrigine with confirmed ANEM showing both eosinophils and rare giant cell infiltrates on endomyocardial biopsy. Although lamotrigine has been reported to cause DRESS, it has not been previously implicated as a cause of ANEM.
Keywords: cardiovascular medicine, cardiovascular system, pathology
Background
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is traditionally treated with steroids and immunosuppressive strategies. However, overcoming complications related to relapses and long-term corticosteroid represents a challenge in treating these patients. This case demonstrates the experimental use of high-dose mepolizumab has the potential to be used as a steroid-sparing option.
Case presentation
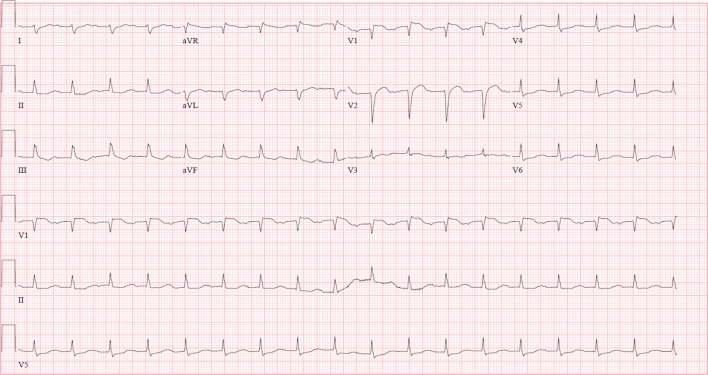
A 45-year-old Caucasian woman with a history of recently diagnosed DRESS syndrome secondary to lamotrigine, was completing a 12-week steroid taper, when she presented with acute chest pain, diaphoresis, dyspnoea on exertion and severe fatigue. She had no fever or rash and an eosinophil count of 270/uL (ref. 0–700/uL). Physical examination revealed a pericardial rub and prominent S4. ECG showed sinus tachycardia with first-degree atrioventricular block and Q-waves in the septal leads with J-point elevation in V1 and non-specific ST-T wave changes (figure 1). Troponin T was 6.81 ng/mL (ref.<0.100 ng/mL). Transthoracic echocardiography (TTE) showed left ventricular systolic dysfunction with global hypokinesis and left ventricular ejection fraction (LVEF) 30%–34%. Cardiac catheterisation revealed normal coronaries. In light of the recent DRESS syndrome, eosinophilic myocarditis was suspected and subsequent cardiac MRI (cMRI) heightened the concern with patchy edema and enhancement of the mid myocardium and subepicardium of the basal lateral, septal and inferior walls (figure 2). A diagnosis of eosinophilic myocarditis was confirmed by right ventricular endomyocardial biopsy with dense eosinophilic infiltrates and myocardial necrosis, but careful pathology review of her slides also detected a few giant cells (figure 3). Our patient was treated with intravenous methylprednisolone 500 mg two times per day and mycophenolate mofetil 1000 mg two times per day in addition to colchicine 0.6 mg two times per day, carvedilol 3.125 mg two times per day and furosemide 20 mg and antibiotic prophylaxis with atovaquone 1500 mg daily and a nystatin swish. She improved clinically and was discharged on prednisone 60 mg orally daily, mycophenolate mofetil 1000 mg two times per day, ciclosporin 100 mg two times per day, carvedilol 3.125 mg two times per day, furosemide 20 mg daily.
Figure 1.
ECG showing sinus tachycardia with first-degree atrioventricular block and Q-waves in the septal leads with J-point elevation in V1 and non-specific ST-T wave changes.
Figure 2.
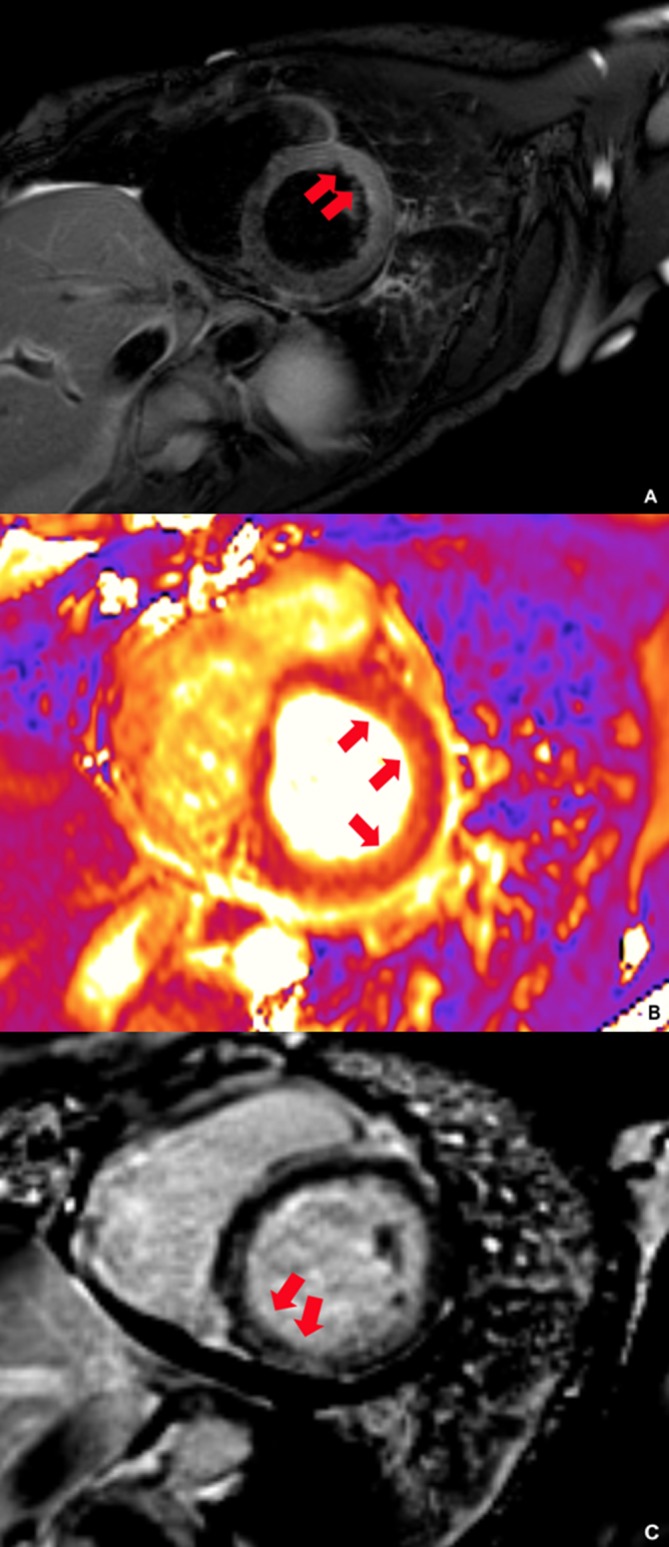
Cardiac MRI. (A) Dark blood image with hyperenhancement of the anterior and lateral cardiac wall (arrows). (B) T2 parametric image (measured at 60 ms) with hyperenhancement of the anterior and lateral walls (arrows). (C) Delayed imaging with patchy enhancement of the mid myocardium and subepicardium of the basal lateral, septal and inferior wall (arrows).
Figure 3.
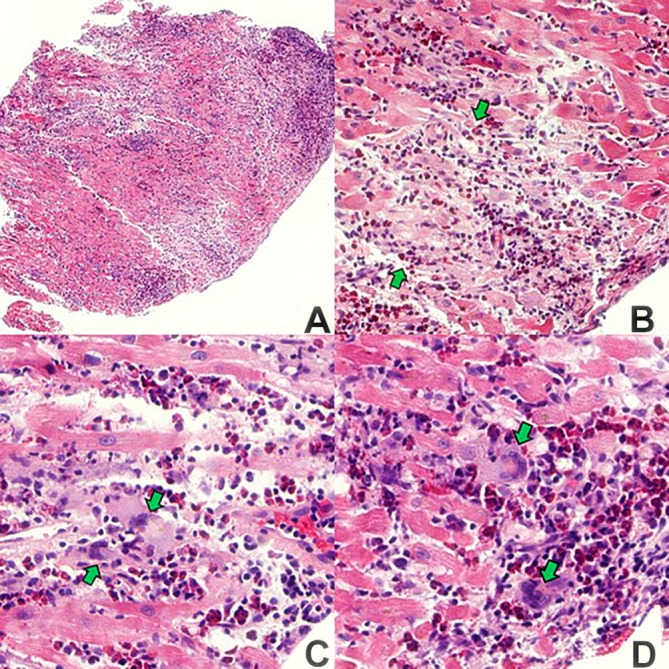
(A) Scanning magnification with dense, patchy, nodular foci of inflammation x25. (B) Focus of myocardial necrosis (demarcated by green arrows). Pallor of necrotic myocytes and dense interstitial inflammation x100. (C) Myocardial fibre necrosis, interstitial inflammation with many eosinophils and two, multinucleated, histiocytic giant cells (green arrows) x200 (D) Two multinucleated histiocytic giant cells (green arrows) and adjacent dense cluster of eosinophils x200.
Two weeks later, her LVEF normalized to 60%. However, at approximately 6 weeks into tapering at a dose of prednisone 30 mg daily, the patient presented with signs of relapse. She developed dyspnoea, a non-pruritic well demarcated, blanchable and erythematous rash across her upper chest (figure 4). There was also buccal mucosa mucositis. A marginally elevated high-sensitive cardiac troponin T (hs-cTnT) 19 ng/mL (ref. 0–14 ng/mL) and pro-brain natriuretic peptide (BNP) 397 pg/mL (ref. 0–299 pg/mL) were detected. TTE calculated an LVEF of 60%–64% and cMRI showed improved biventricular systolic function with persistent patchy residual myocardial edema—though overall improvement. As a result, tapering was suspended and her steroids were maintained at the current dose. An anti-interleukin-5 (IL-5) inhibitor, mepolizumab, was initiated as a steroid-sparing immunosuppressant agent. She was started on monthly intravenous injections at a dose of 300 mg for 3 months. After 3 months, the dose was increased to 500 mg monthly injections. At this dose, the patient has remained asymptomatic and has been able to discontinue her mycophenolate while maintaining her ciclosporin. An extremely slow taper of prednisone at 2.5 mg, every other week has resulted in no adverse signs or symptoms of relapse.
Figure 4.
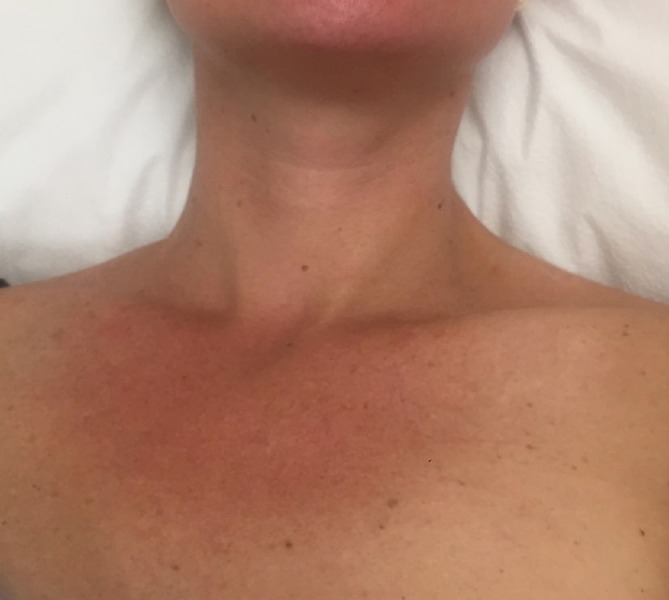
Clinical image revealing erythematous rash across the patient’s upper chest.
Outcome and follow-up
Approximately 1 year after initially presenting with chest pain, the patient has successfully been able to slowly withdraw from her corticosteroid use. While not fully understood, it is our belief that the use of mepolizumab, as a steroid-sparing agent, may have contributed to this outcome as previous attempts to withdraw immunosuppression have resulted in clinical signs of relapse. Other than fatigue, the patient has been asymptomatic with no signs of recurrence. Due to the nature of this disease, the patient continues to be closely monitored with regular follow-up.
Discussion
DRESS syndrome is a characterized by erythema, fever, lymphadenopathy, eosinophilia and organ involvement. A rare complication of this condition is acute necrotising eosinophilic myocarditis (ANEM), which reports have suggested this form of myocarditis develops rapidly and typically leads to poor outcomes, with mortality rates above 50%.1 2 ANEM with giant cells is a rare and an under-recognized complication that can present long after the initial diagnosis of DRESS syndrome. It is characterized by an inflammatory eosinophilic infiltrate that induces myocyte necrosis. Treatment strategies depend on immunosuppressive strategies using corticosteroids with a slow taper while monitoring for signs of relapse.2
The pathology in this case revealed myocarditis characterized by dense, myocardial interstitial inflammation with a predominance of eosinophils. During the close review, focal myocardial necrosis with rare multinucleated histiocytic giant cells was identified. While the presence and role of giant cells in ANEM need further investigation, it is generally accepted to indicate a more advanced disease along a spectrum from indolent to aggressive clinical presentations.3
Treatment regimens have been based on immunosuppression, but are evolving because of the advent of more targeted therapies that inhibit eosinophils.3–5 Aggressive immunosuppression and the use of leading indicators such as hs-cTnT and pro-BNP was the treatment strategy for our patient. We initially chose ciclosporin to aid with steroid tapering. A slow taper was chosen due to the potential of relapsing DRESS symptoms. With signs of relapse and the potential complications of long-term high-dose corticosteroids, the use of an anti-IL-5 antibody was explored. Anti-IL-5 antibodies have been suggested as a steroid-sparing option in patients with hypereosinophilic syndromes for their ability to selectively inhibit eosinophilic production.4 5 Mepolizumab has previously been used as a steroid-sparing therapy in a group of patients with eosinophilic granulomatosis with polyangiitis. In this study, several patients were able to reduce their steroid dependency and obtain remission with mepolizumab at of dose of 300 mg every 4 weeks.5 Of note, the eosinophil count in our patient was only elevated when she was initially diagnosed with DRESS syndrome and may have been suppressed by corticosteroids.
This case highlights the importance of a slow steroid taper in the treatment of ANEM related to DRESS syndrome, and that the use of ciclosporin and mepolizumab has enabled our patient to taper her steroids to doses that had previously resulted signs of relapse. Furthermore, hs-cTnT is valuable, as an indicator of disease activity, it guided steroid taper rate while balancing the risk of complications from long-term, high-dose steroid use against the high mortality risk in ANEM with inadequate disease suppression. Novel therapies that target eosinophils, such as high-dose mepolizumab, are intuitive and may allow further steroid tapering, but are certainly not established therapy. Our patient has done well with close monitoring of clinical progress and the aforementioned serum and radiological indicators, by an interdisciplinary team. We believe the development of registries for rare conditions like ANEM will help achieve treatment regimen consensus which will help improve clinical outcome.
Learning points.
Acute necrotizing eosinophilic myocarditis (ANEM) is an uncommon, but potentially fatal manifestation of relapsing drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome.
Aggressive immunosuppression is the reported treatment strategy in patients with DRESS syndrome and ANEM.
Although further investigation is warranted, mepolizumab is a potential novel steroid-sparing strategy in patients with ANEM.
Serial high-sensitive cardiac troponin T is a useful indicator of disease activity and marker of relapse.
The presence of giant cells in ANEM is a rare occurrence and is generally understood to represent a more aggressive form of eosinophilic myocarditis.
Footnotes
Contributors: RK contributed to the writing and data and image collection of this manuscript. MP contributed to the revision to this work. WT contributed to the collection of images and to revisions. SD contributed in managing patient care and making contributions to revisions.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bourgeois GP, Cafardi JA, Groysman V, et al. . A review of DRESS-associated myocarditis. J Am Acad Dermatol 2012;66:e229–36. 10.1016/j.jaad.2010.11.057 [DOI] [PubMed] [Google Scholar]
- 2.Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part II. Management and therapeutics. J Am Acad Dermatol 2013;68:709.e1–9. 10.1016/j.jaad.2013.01.032 [DOI] [PubMed] [Google Scholar]
- 3.Sabatine MS, Poh KK, Mega JL, et al. . Case records of the Massachusetts General Hospital. Case 36-2007. A 31-year-old woman with rash, fever, and hypotension. N Engl J Med 2007;357:2167–78. 10.1056/NEJMcpc079030 [DOI] [PubMed] [Google Scholar]
- 4.Rothenberg ME, Klion AD, Roufosse FE, et al. . Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med 2008;358:1215–28. 10.1056/NEJMoa070812 [DOI] [PubMed] [Google Scholar]
- 5.Wechsler ME, Akuthota P, Jayne D, et al. . Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. N Engl J Med 2017;376:1921–32. 10.1056/NEJMoa1702079 [DOI] [PMC free article] [PubMed] [Google Scholar]