Abstract
Context
Although important advances have been made in understanding the genetics of endocrine tumors, cellular physiology is relatively understudied as a determinant of tumor behavior. Oxidative stress and reactive oxygen species are metabolic factors that may affect tumor behavior, and these are, in part, controlled by manganese-dependent superoxide dismutase (MnSod), the mitochondrial superoxide dismutase (encoded by SOD2).
Objective
We sought to understand the role of MnSod in the prognosis of aggressive human endocrine cancers and directly assessed the effect of MnSod under- or overexpression on tumor behavior, using established mouse thyroid cancer models.
Methods
We performed transcriptome analysis of human and mouse models of endocrine cancer. To address the role of Sod2 in endocrine tumors, we introduced a Sod2 null allele or a transgenic Sod2 overexpression allele into mouse models of benign thyroid follicular neoplasia or aggressive, metastatic follicular thyroid cancer (FTC) and monitored phenotypic changes in tumor initiation and progression.
Results
In the thyroid, SOD2/Sod2 was downregulated in FTC but not papillary thyroid cancer. Reduced expression of SOD2 was correlated with poorer survival of patients with aggressive thyroid or adrenal cancers. In mice with benign thyroid tumors, Sod2 overexpression increased tumor burden. In contrast, in mice with aggressive FTC, overexpression of Sod2 reduced tumor proliferation and improved mortality rates, whereas its deficiency enhanced tumor growth.
Conclusion
Overall, our results indicate that SOD2 has dichotomous roles in cancer progression and acts in a context-specific manner.
Transcriptome analysis of human endocrine cancers and in vivo genetic manipulation of Sod2 in mouse models demonstrated that Sod2 acts as a tumor suppressor or promoter, depending on tumor context.
Aggressive endocrine cancers are uncommon, and they present therapeutic challenges because of their limited response to conventional chemotherapeutic agents. Thyroid cancer is the most common endocrine malignancy, with >55,000 new cases diagnosed per year in the United States (1). Epithelial thyroid cancers (also known as nonmedullary thyroid cancers) comprise the more common papillary thyroid cancer (PTC), the less common follicular thyroid cancer (FTC), and the rare and highly aggressive anaplastic thyroid cancer (ATC). Although most cases of thyroid cancer are treatable and have a good prognosis, once the tumor has undergone metastasis, overall survival drops significantly because treatment options are limited (2). In contrast, adrenocortical carcinoma (ACC) is a rare cancer, with an incidence of approximately one per million in the United States (3). The prognosis of ACC remains poor, with a 5-year survival rate of <10% for those with advanced disease.
These endocrine tumors, like normal hormone-producing cells, exhibit high metabolic activity; thus, there has been a renewed interest in understanding the metabolic contribution to tumor behavior, particularly as it applies to the transition between normal and neoplastic tissue. In most tissues, energy needs are supplied by aerobic energy generation through the mitochondrial Krebs cycle. In tumors, ATP generation often relies on the glycolytic pathway, and this energetic transition is known as the Warburg effect (4). Because of altered tumor metabolism, most tumors are thought to exhibit enhanced oxidative stress (OS) due to inactivation or dysregulation of mitochondrial enzymes (5). Accumulation of reactive oxygen species (ROS) can lead to OS and cause multiple types of damage to the cell, including ROS-mediated DNA damage (i.e., mutagenesis) and oxidation of cellular proteins and lipids. ROS can also serve a signaling function (6–12), which may be either cell autonomous or through effects on the tumor microenvironment (13–15).
The association of OS with thyroid cancer progression has been suggested by the finding of low total antioxidant levels and an altered OS index in the serum of patients with thyroid cancer, often accompanied by evidence for DNA damage and lipid peroxidation (16–18). However, the mechanistic link between OS and thyroid neoplasia remains poorly understood (19), because studies associating OS with thyroid disorders and cancer have relied on clinical retrospective studies (20). Although the adrenal cortex is also a highly metabolically active tissue, there are no studies to date, to our knowledge, that have evaluated the effect of ROS on tumor behavior.
ROS scavenging in tissue relies heavily on manganese-dependent superoxide dismutase, (MnSod), a mitochondrial enzyme encoded by the nuclear SOD2 gene. MnSOD converts superoxide radicals to hydrogen peroxide (H2O2), which is subsequently detoxified to water by catalase and glutathione peroxidases (GPXs). SOD2 is essential for organism survival: Homozygous knockout (KO) of the murine gene (Sod2) causes embryonic lethality (21, 22), in contrast to the viability of animals with KOs of other members of the SOD family, cytosolic Sod1 and extracellular Sod3 (23, 24).
In this study, we investigated the role of SOD2/Sod2 in thyroid and adrenal cancers using human and mouse mRNA expression datasets. We report that FTCs exhibit decreased expression of SOD2/Sod2, whereas no change is seen in PTCs. Prognostically, reduced levels of SOD2 predicted a poorer survival in ATC and ACC. To address directly Sod2’s role in cancer behavior, we analyzed the effect of its over- or underexpression on the behavior of mouse models of FTC. The results of this study indicate that MnSod exhibits context-specific effects, where increased levels exacerbate tumors of low aggressiveness but are protective in advanced cancers.
Materials and Methods
Transcriptome analysis
The Cancer Genome Atlas and Gene Expression Omnibus was used to screen SOD2 expression in human endocrine cancers. A panel of OS genes was selected from microarray data, with a P value cutoff at 0.01 considered significant. OS genes were selected from previously published microarray data for human and murine FTC (Figs. 1A and 2) (25, 26). The data set in Fig. 1A was downloaded from the European Molecular Biology Laboratory ArrayExpress repository (www.ebi.ac.uk/arrayexpress) using accession identifier E-MEXP-97 (27). Kaplan-Meier curves were analyzed from ATC and ACC data sets, using low and high expression of SOD2 at median cutoff (28). Data sets for Fig. 1B–1D are from The Cancer Genome Atlas (TCGA) thyroid carcinoma (29) [Gene Expression Omnibus accession no. GSE76039 (30)] and the data set for Fig. 1E is from TCGA ACC (28).
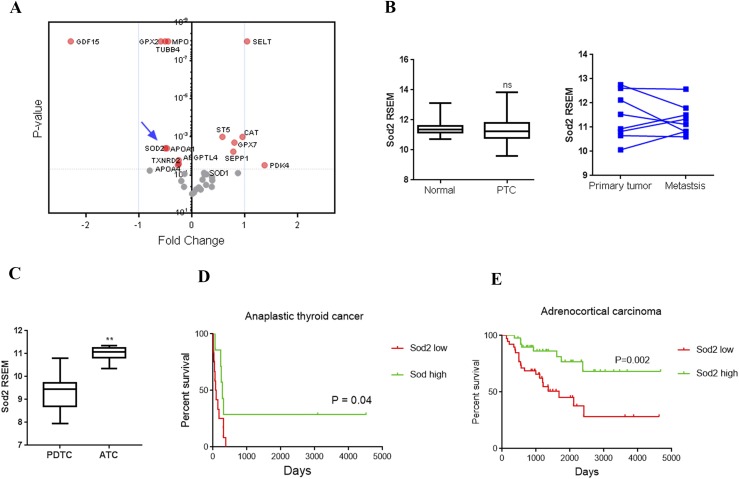
Figure 1.
OS pathways in human endocrine neoplasia. Oxidative pathway and Sod2 expression was analyzed in different endocrine tumors. (A) Deregulation of OS genes in FTC compared with follicular adenoma, analyzed from microarray data. Significantly altered genes above cutoff (P < 0.01) are indicated by red circles; those below cutoff are indicated by gray circles; Sod2 is indicated by an arrow. (B) Expression of Sod2 in PTC compared with normal tissue (left), and matched primary tissue vs metastatic tissue (right). (C) Analysis of Sod2 expression in ATC compared with poorly differentiated thyroid cancer. (D) Kaplan-Meier survival curves of data from patients with ATC, based on low (red line) and high (green line) expression. (E) Kaplan-Meier survival curves of data from patients with ACC based on low (red line) and high (green line) expression. P value was calculated by log-rank test in survival analysis. Sources of microarray data for these analyses are described in Methods. **P < 0.01. ns, not significant; RSEM, RNA-sequencing by expectation-maximization.
Animal Studies
All animal experimentation described was conducted in accordance with accepted standards of humane animal care and in compliance with Ohio State University Institutional Animal Care and Use Committee regulations. We have previously described the use of the thyroid-specific Tpo-cre line (31) for the generation of mice lacking Pten in the thyroid, either by itself (Pten-TpoKO) or in combination with deletion of Prkar1a [double R1a-Pten KO (DRP-TpoKO)] (25). To create mice lacking one copy of Sod2 (i.e., haploinsufficiency), these mice were crossed independently with animals heterozygous for a null allele of Sod2 (stock no. 002973; Jackson Laboratories) (32). The resulting mice were backcrossed to the respective parental thyroid-specific KO lines to generate Sod2+/− Pten-TpoKO and Sod2+/− DRP-TpoKO mice. To analyze the effect of overexpression of Sod2, we took a similar approach to integrate the Sod2-Tg allele (33) (in the hemizygous state), resulting in two lines with Sod2 overexpression: Sod2-Tg Pten-TpoKO and Sod2-Tg DRP-TpoKO mice. Because the thyroid KO lines were in an FVB/N-strain background and the Sod2 alleles were in the C57Bl/6J-strain background, all animals in this study were littermates of a mixed background. Animals from both sexes were analyzed and found to exhibit the same characteristics (data not shown). Thus, the data presented here include aggregated information from male mice and female mice.
Ultrasonography
Three-dimensional thyroid ultrasonography was performed at 3-month intervals on live animals, using a VisualSonics Vevo 2100 ultrasound platform equipped with an MS550D transducer. Images were acquired in three-dimensional mode and volumes calculated using Vevolab 2.1 software in a blinded fashion.
Histology
Dissected mouse thyroid tissues were fixed in 10% buffered formalin solution and embedded in paraffin. Tissue sections (5 µm) were stained with hematoxylin and eosin. Immunohistochemistry was performed and results quantified as previously described (34), with antibodies for Ki-67 (catalog no. ab15580; Abcam) or MnSod (catalog no. PA1-125; Thermo Fisher Scientific).
Statistics
All data, except Kaplan-Meier curves and cancer incidence, were analyzed via paired or unpaired t test using Prism GraphPad software. Thyroid volume analysis was done using a linear mixed model among three different genotype groups [Sod2-wt (wild-type), Sod2+/−, and Sod2-tg] averaged across months and sex. Holm procedure was used to adjust for multiple comparisons. P < 0.05 was considered significant. For microarray analysis of OS genes, P < 0.01 was considered significant. Log-rank test was used for analyzing Kaplan-Meier curves.
Results
Clinical relevance of altered OS status in human endocrine cancers
SOD2 gene expression exhibits complex patterns with downregulation in some cancers and upregulation in others, but a comprehensive picture of thyroid carcinoma in regard to OS status and SOD2 expression is lacking (8, 35, 36). Given the importance of ROS signaling on thyroid function, we examined publicly available and previously published data sets examining mRNA expression patterns in thyroid cancer (28–30, 37). PTC is the most common type of thyroid cancer and generally has a good prognosis. FTC is more likely to undergo hematogenous spread than PTC (38), whereas ATC is extremely aggressive with frequent metastases and a median survival of 3 to 5 months (39, 40). Analysis of OS genes in previously published human microarray data revealed that the oxidative pathway was altered in FTC compared with follicular adenoma (FA; Fig. 1A) (27). Expression of SOD2 was downregulated in FTC (fold change, 0.7; P < 0.001) compared with FA. Contrary to other types of cancers, analysis of PTC from TCGA data revealed that SOD2 was neither significantly altered in the primary tumor nor in metastatic tissue when compared with normal and matched primary tumor, respectively (41, 42) (Fig. 1B). However, this could be a limitation of the number of available samples with metastases. When the TCGA data were broken out by driver mutation, we observed that BRAF mutant cancers (which formed the majority of samples in the cohort) had SOD2 levels similar to wild-type, whereas RAS mutant tumors had reduced SOD2 levels, similar to those observed in FTC. Tumors lacking mutations in either of these genes exhibited intermediate SOD2 levels (Supplemental Fig. 1). In contrast, in more aggressive anaplastic cancer, expression of SOD2 was significantly increased compared with that is poorly differentiated thyroid cancer (Fig. 1C) (30).
To determine if SOD2 levels correlated with outcomes, we studied overall survival in patients with ATC (30), because a paucity of deaths in PTC and FTC cohorts precluded this type of approach (27, 29). In this analysis, a higher SOD2 level correlated with significantly increased survival in the ATC cohort (Fig. 1D). To further test if SOD2 expression influenced survival outcomes in other aggressive endocrine cancers, we evaluated the TCGA data for ACC, another endocrine cancer with poor overall outcome (28). We observed that patients with high SOD2 expression had increased survival, whereas low SOD2 levels poorly correlated with survival status in ACC (Fig. 1E).
Altered OS status in mouse thyroid cancer models
Frequent MnSOD dysregulation in cancers suggests that the enzyme may play an important role in cancer progression and metastasis (43, 44). According to some studies, SOD2 shows a positive correlation with aggressive cancer phenotypes (44–47). Therefore, we investigated the relationship between Sod2 expression and histopathological features, using mouse models of FTC. Previous mouse modeling data from our laboratory have shown that the activation of Akt (arising from Pten ablation) results in FAs, whereas activation of PKA signaling [arising from loss of Prkar1a (R1a)] is sufficient to promote a locally invasive FTC phenotype (25). When there is dual activation of these pathways (via combined loss of R1a and Pten), mice develop an aggressive FTC, which exhibits metastatic behavior similar to that seen in human FTCs. Thus, we used these FTC disease-progression mouse models to analyze the OS in the FAs in Pten-TpoKO (70% incidence), in the locally invasive FTCs in R1a-TpoKO (20% FA, 80% FTC), and in 100% penetrant, metastatic FTC in DRP-TpoKO mice.
We analyzed the expression of genes in OS pathways in our FTC disease progression mouse models by mining previously published microarray data (25). As expected, the proportion of significantly altered genes in the OS pathway was higher in the more aggressive DRP-TpoKO model than in the R1a-TpoKO and Pten-TpoKO models (Fig. 2A–2C). mRNA expression of Sod2 was significantly reduced in DRP-TpoKO and R1a-TpoKO but not in Pten-TpoKO mice (25, 48). Thus, similar to the human FTC data shown in Fig. 1, Sod2 expression was unchanged in adenomas and downregulated in aggressive types of murine thyroid cancer models (Fig. 2D).
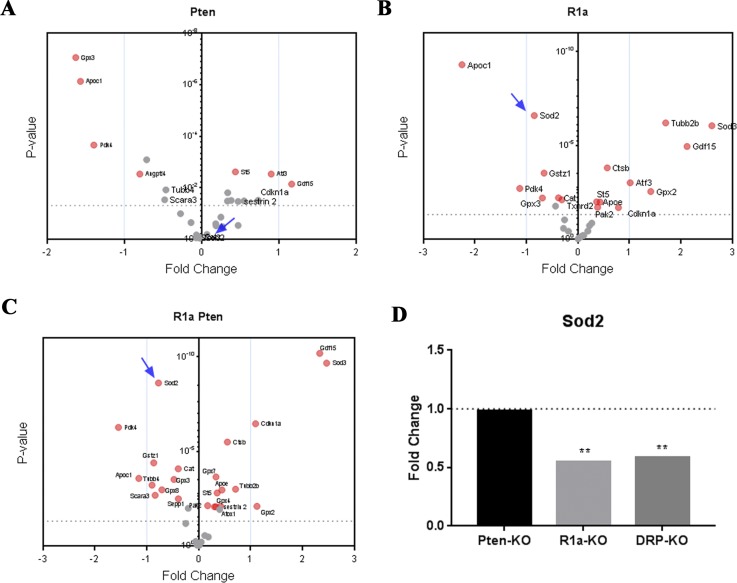
Figure 2.
Deregulation of OS genes in murine tumor progression models. Microarray data comparing Pten-, R1a-, and DRP-TpoKO tumors. Oxidative pathways genes were analyzed in (A) Pten-TpoKO tumors, (B) R1a-TpoKO tumors, and (C) DRP-TpoKO tumors. Significantly altered genes above cutoff (P < 0.01) are indicated by red circles; those below the cutoff are indicated by gray circles; Sod2 is indicated by the arrow in panels A–C. Wild-type Cre-negative littermates were used for comparison for each model. (D) Expression of Sod2 in each tumor model compared with wild-type control. Dotted line represents the fold change of Cre-negative control tissue for each group. **P < 0.01.
Furthermore, the ratio of Sod2 to H2O2-scavenging enzyme levels is an important biomarker of tumor progression (49). Our microarray analysis revealed that Pten-TpoKO mice had a Sod2-to-GPx1 ratio of 2.7 and Sod2-to-Catalase ratio of 1. R1a-TpoKO mice had Sod2-to-GPx1 ratios of 0.53 and Sod2-to-Catalase of 0.78. Similar to R1a-null mice, the Sod2-to-GPx1 and Sod2-to-Catalase ratios were 0.65 and 0.78, respectively, in DRP-TpoKO tumors. As Sod2 was downregulated in R1a-TpoKO and DRP-TpoKO thyroid tumors, the expression of the extracellular superoxide dismutase Sod3 was significantly upregulated. However, Sod3 is an extracellular enzyme, so its role in detoxifying the intracellular compartment is unclear (50). Overall, SOD2/Sod2 genes were similarly downregulated in human FTC and murine FTC models, but not in murine adenomas or human PTC. The OS gene signature as well as ratios of antioxidant enzymes indicate a progression of gene expression changes as the tumors become more aggressive, thus setting the stage for OS stress in different thyroid carcinoma models.
Effects of over- and underexpression of Sod2 in murine models of thyroid follicular neoplasias
OS is proposed as an important factor in thyroid cancer; however, the expression of SOD2 has been suggested to correlate with the degree of differentiation (51). Thus far, to our knowledge, the effect of Sod2 manipulation has not been experimentally investigated in endocrine cancer models. To this end, we genetically manipulated OS in our FTC mouse models as described in Methods by either over- or underexpressing the gene. Because Sod2−/− mice are not viable, we used Sod2+/− mice to achieve a reduction in Sod2, because these animals exhibit about 50% of the MnSod activity of the wild-type mice (52). We used a transgenic line overexpressing Sod2 globally as a means to upregulate expression of the enzyme (33). Notably, Sod2+/− mice have significantly elevated measures of OS (21, 52–56) and increased cancer incidence (54), whereas Sod2-Tg mice have enhanced mitochondrial oxidative capacity and decreased cancer incidence (33, 57). Pten- and DRP-TpoCre mice were bred with Sod2+/− and Sod2-Tg animals to generate thyroid-specific tumors with up- or downregulation of MnSod as a means to understand the effect of altered OS in FTC disease progression.
The mice were analyzed by ultrasonography at 3-month intervals for up to 1 year to record thyroid growth over time. At study end point, mice were subjected to histological analysis to analyze incidence of cancer. For baseline comparison, control groups with wild-type Sod2 (i.e., Pten-TpoKO or DRP-TpoKO) were used. The expression of MnSod was confirmed in Sod2-wt underexpression and overexpression models by immunohistochemistry (Supplemental Fig. 2) and by quantitative real-time PCR of thyroid mRNA for Sod2 (data not shown).
We analyzed the effect of Sod2 overexpression (Sod2-Tg) and underexpression (Sod2+/−) in Pten-TpoKO mice, which exhibit FAs. Manipulation of Sod2 activity in this model produced no change in mouse survival up to 1 year of age (data not shown). However, ultrasound analysis revealed that Sod2-Tg consistently increased the tumor volume, whereas Sod2+/− did not affect gross tumor size of Pten-TpoKO mice (Fig. 3A). Consistent with increased tumor size, Pten-TpoKO mice with Sod2-Tg had a significantly higher incidence of carcinoma, compared with Sod2-wt or Sod2+/− mice (χ2P = 0.007; Fig. 3B). One Sod2-Tg Pten-TpoKO mouse developed lung metastasis, whereas none of the other groups exhibited lung metastasis. Hence, higher expression of Sod2 correlated with advanced cancer in mice with the Pten-KO background. There were no sex differences in thyroid size in Pten-TpoKO animals (data not shown). Interestingly, when Sod2 levels were manipulated in this setting (either increased or decreased), male mice had a trend toward higher tumor volume than females (overall P = 0.053). However, when corrected for body weight, this trend disappeared.
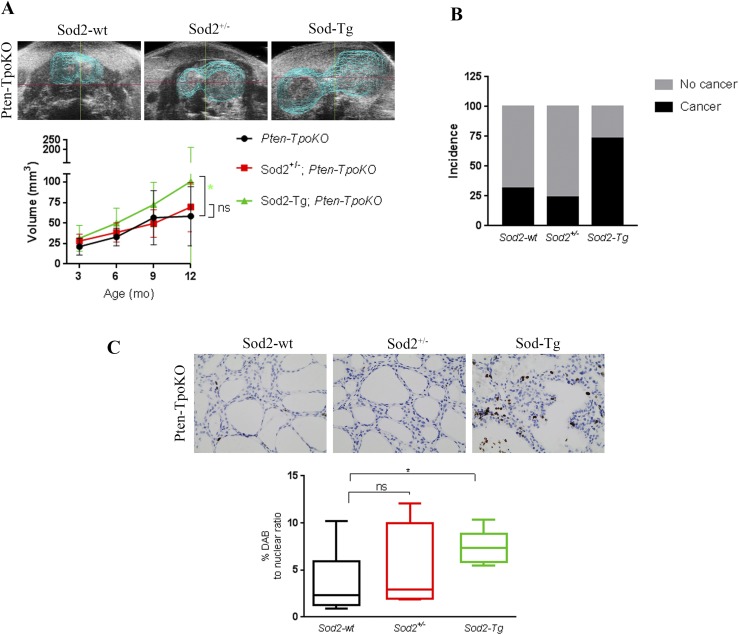
Figure 3.
Sod2 overexpression increases tumor aggressiveness in a Pten KO mouse model of FA. (A, top) Three-dimensional rendering of ultrasonographic images of Pten-TpoKO with Sod2-wt, Sod2+/− and Sod2-Tg at 12 months. (A, bottom) Average thyroid volumes determined by three-dimensional ultrasonography at 3, 6, 9, and 12 mos in Pten-TpoKO mice with Sod2-wt (black line; n = 12), Sod2+/− (red line; n = 22), and Sod2-Tg (green line; n = 18). (B) The incidence of thyroid carcinoma in Pten-TpoKO mice with Sod2-wt, Sod2+/−, and Sod2-Tg. (C, top) Representative ×40 images of Ki67 staining in thyroid tumors of mice at 12 mos of age. (C, bottom) Quantification of proliferation represented as percent DAB to nuclear ratio. Graphs present mean data ± SD. *P ≤ 0.05. DAB, diaminobenzidine.
To better understand the role Sod2 plays in tumor size in the Pten-TpoKO mice, we examined proliferation markers in the tissue. Sod-Tg animals exhibited significantly increased proliferation as evidenced by Ki-67 staining compared with controls (Fig. 3C). Proliferation in Sod2+/− Pten-TpoKO mice was not altered compared with Pten-TpoKO alone.
Effects of over- and underexpression of Sod2 in metastatic FTC model
We next analyzed the effect of Sod2 manipulation in a more aggressive tumor model. We have shown that DRP-TpoKO mice form aggressive thyroid tumors with capsular fibrosis and local invasion, and have the ability to form distant metastases (25). In this model, Sod2 deficiency consistently promoted tumor growth and overexpression reduced tumor growth (Fig. 4A). All three groups of mice developed carcinomas with 100% penetrance, but the gross tumor size in Sod2+/− DRP-TpoKO mice was increased compared with that of controls (P = 0.06) and was significantly higher than in Sod2-Tg DRP-TpoKO mice (P = 0.006). A 12-month time point was omitted because few mice survived to this age, although genotype played an important role in this observation: The proportion of DRP-TpoKO mice surviving at 9 months was 26% in the Sod2-wt group, 15% in the Sod2-deficient group, and 60% in the Sod2-overexpression group. Overall, thyroid tumors of Sod2+/− DRP-TpoKO mice had nonsignificantly increased proliferation compared with those of control mice (P = 0.24). The reduced tumor size in Sod2-Tg; DRP-TpoKO was supported by the finding of significantly decreased proliferation in these tumors, as evidenced by Ki67 staining (Fig. 4C). Moreover, Sod2-Tg DRP-TpoKO mice had significantly longer lifespans (median survival of 52 weeks compared with 23 weeks in control mice) despite having similar cancer incidence, whereas Sod2+/− DRP-TpoKO genotype did not seem to worsen the lifespan of mice (Fig. 4C). We hypothesized that loss of Sod2 might correlate with advanced disease progression and metastasis, hence we screened lungs of these mice to check for metastatic spread. The frequency of lung metastases in all groups of DRP-TpoKO mice was similar (Fig. 4D). There was no sex effect in the DRP-TpoKO mice (data not shown).
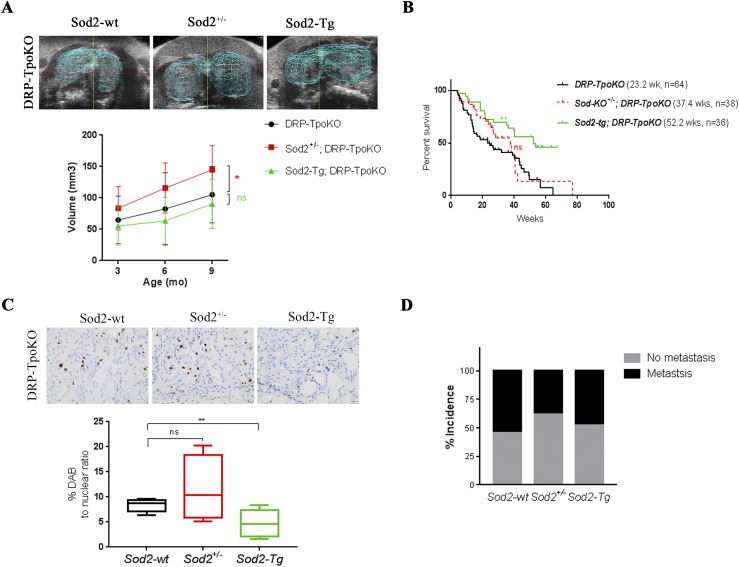
Figure 4.
Sod2 deficiency induces tumor growth in a dual Pten/Prkar1a KO mouse model of metastatic FTC. (A, top) Three-dimensional rendering of ultrasonographic images of thyroid glands in DRP-TpoKO with Sod2-wt, Sod2+/−, and Sod2-Tg mice at 12 mos. (A, bottom) Average thyroid volumes determined by three-dimensional ultrasonography at 3, 6, 9, and 12 mos in DRP-TpoKO mice with Sod2-wt (black line; n = 16), Sod2+/− (red line; n = 12), and Sod2-Tg (green line; n = 20). (B) Kaplan-Meier survival curves of DRP-TpoKO mice with Sod2-wt (black line), Sod2 deficiency (red line), and Sod2 overexpression (green line). (C, top) Representative ×40 images of Ki67 staining in thyroid tumors of mice at 12 mos of age. (C, bottom) Quantification of proliferation represented as percent DAB to nuclear ratio. (D) Percent incidence of lung metastases in DRP-TpoKO mice with Sod2-wt, Sod2+/−, and Sod2-Tg. *P ≤ 0.05; **P ≤ 0.01. DAB, diaminobenzidine.
Discussion
Alterations in the cellular response to OS have been proposed to affect the behavior of cancers, including previous studies of thyroid cancer (16, 19). Hormone-secreting endocrine tumors have high metabolic activity and thus may be especially sensitive to metabolic changes. Furthermore, the thyroid and adrenal glands have unique requirements for the handling of ROS because hormone generation requires the action of specific peroxidases (e.g., thyroid peroxidase) and cytochrome oxidases (e.g., multiple P450 family members). However, to our knowledge, the role of mitochondrial antioxidants in thyroid and adrenal cancer progression has been unexplored. Mitochondria are the main site of ROS production; thus, the MnSOD is assumed to be the major antioxidant enzyme, and we have focused on this enzyme in this report.
In analysis of SOD2/Sod2 mRNA expression in human and mouse differentiated thyroid cancer, we observed a consistent downregulation of the gene in FTC. These transcriptional changes were not observed in human or mouse FAs, suggesting that the change is correlated with cellular transformation. Interestingly, PTC tumors in the TCGA data set did not exhibit overall alterations in SOD2, consistent with a different molecular behavior of these tumors. This effect was specific for BRAF mutant tumors, because RAS mutant tumors had a profile similar to FTCs, providing another point of evidence connecting genotype to molecular phenotype, rather than histological phenotype, as has been discussed by others (58). SOD2 levels were increased as tumors became less differentiated, with ATCs having the highest levels. We also found SOD2 transcript levels to be predictive of tumor outcome in ATC: Reduced transcript levels were associated with poorer outcome.
To see if these observations were generalizable to other endocrine cancers, we analyzed a set of human ACC data to look for a correlation between SOD2 levels and patient survival. We chose to study ACC because the adrenal gland is another endocrine organ that relies heavily on the cAMP/PKA pathway for trophic growth and physiologic hormone secretion. As in the analysis of aggressive thyroid cancers, ACCs that expressed higher levels of SOD2 were associated with improved patient survival.
Because good mouse models for adrenal cancer do not exist, we focused on modeling the effects of Sod2 in FTC, where existing mouse models enabled us to assess directly the effects of manipulating Sod2 levels on tumor behavior. Sod2 overexpression in FA (Pten-null mice) led to a more aggressive cancer phenotype, which was accompanied by increased thyrocyte proliferation. In contrast, the same increase in Sod2 reduced cell proliferation in the metastatic FTC model (R1aPten-null mice). Although tumor size was decreased (but did not reach statistical thresholds) and there was no reduction in metastases, the mice had increased survival, pointing to a less aggressive cancer phenotype. When Sod2 levels were decreased in this model, tumors were larger, with a trend toward enhanced proliferation, although there was no change in overall survival of the animals. Mortality in these mice is largely due to upper aerodigestive tract obstruction by the large thyroid tumors. We would expect that if tumors underwent resection to relieve the obstruction (as would occur in human patients), we would see the survival benefit of higher Sod2 levels, as seen in the human data.
On the basis of these observations, we conclude that the effects of MnSOD alteration are context dependent and complex. Specifically, increasing Sod2 expression promotes tumors of low aggressiveness, whereas it suppresses those with high aggressiveness. The study presented here was designed to address directly the effects of manipulating Sod2 in altering tumor behavior, and, to our knowledge, it is the first to demonstrate these dichotomous effects using genetically engineered mouse tumor models with intact immune systems. Future studies will be required to fully determine the mechanism of these observations; however, studies from other systems provide valuable insight into the dichotomous effects of Sod2 manipulation (59–61), which has recently been reviewed (62).
In prostate cancer, increased MnSod expression is associated with a strongly increased risk of tumor formation, likely due to MnSod’s function as a peroxidase. Increased intracellular peroxide leads to enhanced mutagenesis, with resultant tumorigenesis (63). It has also been shown that peroxide accumulation causes activation of protumorigenic and proangiogenic pathways, including AKT, HIF1α, and VEGF (48, 64, 65). There also are data suggesting that SOD2 enhances lung tumor aggressiveness through activation of NF-kB (66). These signaling alterations may explain how indolent tumors, which often lack important activation of these pathways, begin to acquire more aggressive features.
In contrast to the findings in benign tumors, aggressive tumors in humans and mice clearly do worse with reduced SOD2/Sod2 expression. A possible explanation for the discrepancy is that aggressive and metastatic tumors have already developed mechanisms to acquire activation of tumorigenic pathways; therefore, the ability to scavenge ROS becomes a protective effect preventing further damage. We observed a reduction in Sod2-to-Gpx1 and Sod2-to-catalase ratios in DRP-TpoKO mice, indicating an inability to scavenge ROS. When Sod2 is increased, better H2O2 removal can suppress cell stress as a means to decrease tumor cell proliferation and improve the overall outcome. This hypothesis was previously tested with aggressive pancreatic cancer and glioma cell lines, in which Sod2 overexpression reduced growth in vitro and in xenografts (67, 68). Furthermore, in a chemical carcinogenesis model of melanoma, Sod2 overexpression reduced tumorigenesis and tumor aggressiveness through a mechanism that involved suppression of oxidative cell damage, which may involve tumor cells and the local microenvironment (69).
A potential confounding factor in these studies is the presence of hyperthyroidism: DRP-TpoKO mice are hyperthyroid, whereas Pten-TpoKO mice are euthyroid (25, 48). However, short-term studies to examine the role of hyper- or hypothyroidism on tumor phenotype did not show any substantial effects (data not shown). We also evaluated the possibility that ROS generation from infiltrating immune cells might bias these data; however, analysis of the mouse tumors did not show evidence for a substantial number of immune cells within the tumors (data not shown).
In conclusion, a growing body of evidence suggests that antioxidant enzymes have crucial roles in cancer development. The results of study of SOD2 gene expression in human thyroid and other endocrine tumors demonstrate the dichotomous effects of this enzyme. In normal tissues or benign neoplasias, increases in SOD2/Sod2 expression seem to promote excess tumor growth; in contrast, in aggressive cancers, increases in SOD2/Sod2 ameliorate tumor burden. The context-dependent changes in SOD2 expression may be linked to changes in expression as tumor cells progress from an initial benign stage to the acquisition of more invasive and metastatic phenotypes, or they may be dependent on the underlying oncogenic drivers (35, 41, 58). Moreover, limited research is available concerning OS and use of antioxidants in thyroid disorders such as hypothyroidism and autoimmune thyroiditis (70, 71); in the case of cancer treatment, the research remains controversial (36, 72, 73). It is evident that further refinement of the understanding of reduction–oxidation reaction balance and the role of superoxide dismutases on tumor development and behavior is needed to avoid potentially risky treatments for patients with cancer.
Supplementary Material
Acknowledgments
The authors thank Dr. Daret St. Clair for providing the Sod2-Tg mouse model and Xiaoli Zhang for statistical analyses.
Financial Support: This work was supported by the National Institutes of Health (Grant P01CA124570 to L.S.K.) and The Ohio State University Comprehensive Cancer Center (Grant P30CA0168058). A.A., D.H., and A.M. were supported in part by individual Pelotonia Fellowship Program grants.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ACC
adrenocortical carcinoma
- ATC
anaplastic thyroid cancer
- DRP-TpoKO
double R1a-Pten knockout
- FA
follicular adenoma
- FTC
follicular thyroid cancer
- GPX
glutathione peroxidase
- H2O2
hydrogen peroxide
- KO
knockout
- MnSOD
manganese-dependent superoxide dismutase
- OS
oxidative stress
- PTC
papillary thyroid cancer
- R1a
Prkar1a
- ROS
reactive oxygen species
- TCGA
The Cancer Genome Atlas
References
- 1. Nguyen QT, Lee EJ, Huang MG, Park YI, Khullar A, Plodkowski RA. Diagnosis and treatment of patients with thyroid cancer. Am Health Drug Benefits. 2015;8(1):30–40. [PMC free article] [PubMed] [Google Scholar]
- 2. Kirschner LS, Qamri Z, Kari S, Ashtekar A. Mouse models of thyroid cancer: a 2015 update. Mol Cell Endocrinol. 2016;421:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ, Hammer GD. Adrenocortical carcinoma. Endocr Rev. 2014;35(2):282–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Potter M, Newport E, Morten KJ. The Warburg effect: 80 years on. Biochem Soc Trans. 2016;44(5):1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44(5):479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harris IS, Blaser H, Moreno J, Treloar AE, Gorrini C, Sasaki M, Mason JM, Knobbe CB, Rufini A, Hallé M, Elia AJ, Wakeham A, Tremblay ML, Melino G, Done S, Mak TW. PTPN12 promotes resistance to oxidative stress and supports tumorigenesis by regulating FOXO signaling. Oncogene. 2014;33(8):1047–1054. [DOI] [PubMed] [Google Scholar]
- 7. Bell EL, Emerling BM, Ricoult SJ, Guarente L. SirT3 suppresses hypoxia inducible factor 1α and tumor growth by inhibiting mitochondrial ROS production. Oncogene. 2011;30(26):2986–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol. 2010;38(1):96–109. [DOI] [PubMed] [Google Scholar]
- 9. Guo YL, Chakraborty S, Rajan SS, Wang R, Huang F. Effects of oxidative stress on mouse embryonic stem cell proliferation, apoptosis, senescence, and self-renewal. Stem Cells Dev. 2010;19(9):1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schieke SM, Ma M, Cao L, McCoy JP Jr, Liu C, Hensel NF, Barrett AJ, Boehm M, Finkel T. Mitochondrial metabolism modulates differentiation and teratoma formation capacity in mouse embryonic stem cells. J Biol Chem. 2008;283(42):28506–28512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bigarella CL, Liang R, Ghaffari S. Stem cells and the impact of ROS signaling. Development. 2014;141(22):4206–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pinegin B, Vorobjeva N, Pashenkov M, Chernyak B. The role of mitochondrial ROS in antibacterial immunity. J Cell Physiol. 2018;233(5):3745–3754. [DOI] [PubMed] [Google Scholar]
- 13. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang J, Lam GY, Brumell JH. Autophagy signaling through reactive oxygen species. Antioxid Redox Signal. 2011;14(11):2215–2231. [DOI] [PubMed] [Google Scholar]
- 15. Costa A, Scholer-Dahirel A, Mechta-Grigoriou F. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin Cancer Biol. 2014;25:23–32. [DOI] [PubMed] [Google Scholar]
- 16. Wang D, Feng JF, Zeng P, Yang YH, Luo J, Yang YW. Total oxidant/antioxidant status in sera of patients with thyroid cancers. Endocr Relat Cancer. 2011;18(6):773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Young O, Crotty T, O’Connell R, O’Sullivan J, Curran AJ. Levels of oxidative damage and lipid peroxidation in thyroid neoplasia. Head Neck. 2010;32(6):750–756. [DOI] [PubMed] [Google Scholar]
- 18. Szarek E, Ball ER, Imperiale A, Tsokos M, Faucz FR, Giubellino A, Moussallieh FM, Namer IJ, Abu-Asab MS, Pacak K, Taïeb D, Carney JA, Stratakis CA. Carney triad, SDH-deficient tumors, and Sdhb+/- mice share abnormal mitochondria. Endocr Relat Cancer. 2015;22(3):345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xing M. Oxidative stress: a new risk factor for thyroid cancer. Endocr Relat Cancer. 2012;19(1):C7–C11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lassoued S, Mseddi M, Mnif F, Abid M, Guermazi F, Masmoudi H, El Feki A, Attia H. A comparative study of the oxidative profile in Graves’ disease, Hashimoto’s thyroiditis, and papillary thyroid cancer. Biol Trace Elem Res. 2010;138(1-3):107–115. [DOI] [PubMed] [Google Scholar]
- 21. Vincent AM, Russell JW, Sullivan KA, Backus C, Hayes JM, McLean LL, Feldman EL. SOD2 protects neurons from injury in cell culture and animal models of diabetic neuropathy. Exp Neurol. 2007;208(2):216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11(4):376–381. [DOI] [PubMed] [Google Scholar]
- 23. Sentman ML, Granström M, Jakobson H, Reaume A, Basu S, Marklund SL. Phenotypes of mice lacking extracellular superoxide dismutase and copper- and zinc-containing superoxide dismutase. J Biol Chem. 2006;281(11):6904–6909. [DOI] [PubMed] [Google Scholar]
- 24. Carlsson LM, Jonsson J, Edlund T, Marklund SL. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc Natl Acad Sci USA. 1995;92(14):6264–6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pringle DR, Vasko VV, Yu L, Manchanda PK, Lee AA, Zhang X, Kirschner JM, Parlow AF, Saji M, Jarjoura D, Ringel MD, La Perle KM, Kirschner LS. Follicular thyroid cancers demonstrate dual activation of PKA and mTOR as modeled by thyroid-specific deletion of Prkar1a and Pten in mice. J Clin Endocrinol Metab. 2014;99(5):E804–E812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marsh DJ, Coulon V, Lunetta KL, Rocca-Serra P, Dahia PL, Zheng Z, Liaw D, Caron S, Duboué B, Lin AY, Richardson AL, Bonnetblanc JM, Bressieux JM, Cabarrot-Moreau A, Chompret A, Demange L, Eeles RA, Yahanda AM, Fearon ER, Fricker JP, Gorlin RJ, Hodgson SV, Huson S, Lacombe D, LePrat F, Odent S, Toulouse C, Olopade OI, Sobol H,Tishler S, Woods CG, Robinson BG, Weber HC, Parsons R, Peacocke M, Longy M,Eng C. Mutation spectrum and genotype-phenotype analyses in Cowden disease and Bannayan-Zonana syndrome, two hamartoma syndromes with germline PTEN mutation. Hum Mol Genet. 1998;7(3):507–515. [DOI] [PubMed] [Google Scholar]
- 27. Weber F, Shen L, Aldred MA, Morrison CD, Frilling A, Saji M, Schuppert F, Broelsch CE, Ringel MD, Eng C. Genetic classification of benign and malignant thyroid follicular neoplasia based on a three-gene combination. J Clin Endocrinol Metab. 2005;90(5):2512–2521. [DOI] [PubMed] [Google Scholar]
- 28. Zheng S, Cherniack AD, Dewal N, Moffitt RA, Danilova L, Murray BA, Lerario AM, Else T, Knijnenburg TA, Ciriello G, Kim S, Assie G, Morozova O, Akbani R, Shih J, Hoadley KA, Choueiri TK, Waldmann J, Mete O, Robertson AG, Wu HT, Raphael BJ, Shao L, Meyerson M, Demeure MJ, Beuschlein F, Gill AJ, Sidhu SB, Almeida MQ, Fragoso MCBV, Cope LM, Kebebew E, Habra MA, Whitsett TG, Bussey KJ, Rainey WE, Asa SL, Bertherat J, Fassnacht M, Wheeler DA, Hammer GD, Giordano TJ, Verhaak RGW; Cancer Genome Atlas Research Network . Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer Cell. 2016;30(2):363. [DOI] [PubMed] [Google Scholar]
- 29. Cancer Genome Atlas Research Network Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159(3):676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP, Xu B, Schultz N, Berger MF, Sander C, Taylor BS, Ghossein R, Ganly I, Fagin JA. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016;126(3):1052–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kusakabe T, Kawaguchi A, Kawaguchi R, Feigenbaum L, Kimura S. Thyrocyte-specific expression of Cre recombinase in transgenic mice. Genesis. 2004;39(3):212–216. [DOI] [PubMed] [Google Scholar]
- 32. Lebovitz RM, Zhang H, Vogel H, Cartwright J Jr, Dionne L, Lu N, Huang S, Matzuk MM. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci USA. 1996;93(18):9782–9787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yen HC, Oberley TD, Vichitbandha S, Ho YS, St Clair DK. The protective role of manganese superoxide dismutase against adriamycin-induced acute cardiac toxicity in transgenic mice. J Clin Invest. 1996;98(5):1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ashtekar A, Huk D, Magner A, La Perle K, Zhang X, Piruat JI, López-Barneo J, Jhiang SM, Kirschner LS. Sdhd ablation promotes thyroid tumorigenesis by inducing a stem-like phenotype. Endocr Relat Cancer. 2017;24(11):579–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dhar SK, Tangpong J, Chaiswing L, Oberley TD, St Clair DK. Manganese superoxide dismutase is a p53-regulated gene that switches cancers between early and advanced stages. Cancer Res. 2011;71(21):6684–6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Le Gal K, Ibrahim MX, Wiel C, Sayin VI, Akula MK, Karlsson C, Dalin MG, Akyürek LM, Lindahl P, Nilsson J, Bergo MO. Antioxidants can increase melanoma metastasis in mice. Sci Transl Med. 2015;7(308):308re8. [DOI] [PubMed] [Google Scholar]
- 37. Aldred MA, Huang Y, Liyanarachchi S, Pellegata NS, Gimm O, Jhiang S, Davuluri RV, de la Chapelle A, Eng C. Papillary and follicular thyroid carcinomas show distinctly different microarray expression profiles and can be distinguished by a minimum of five genes. J Clin Oncol. 2004;22(17):3531–3539. [DOI] [PubMed] [Google Scholar]
- 38. Nguyen XV, Roy Choudhury K, Tessler FN, Hoang JK. Effect of tumor size on risk of metastatic disease and survival for thyroid cancer: implications for biopsy guidelines. Thyroid. 2018;28(3):295–300. [DOI] [PubMed] [Google Scholar]
- 39. Song YS, Jung CK, Jung KC, Park YJ, Won JK. Rare manifestations of anaplastic thyroid carcinoma: the role of BRAF mutation analysis. J Korean Med Sci. 2017;32(10):1721–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Besic N, Gazic B. Sites of metastases of anaplastic thyroid carcinoma: autopsy findings in 45 cases from a single institution. Thyroid. 2013;23(6):709–713. [DOI] [PubMed] [Google Scholar]
- 41. Termini L, Fregnani JH, Boccardo E, da Costa WH, Longatto-Filho A, Andreoli MA, Costa MC, Lopes A, da Cunha IW, Soares FA, Villa LL, Guimarães GC. SOD2 immunoexpression predicts lymph node metastasis in penile cancer. BMC Clin Pathol. 2015;15(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ye H, Wang A, Lee BS, Yu T, Sheng S, Peng T, Hu S, Crowe DL, Zhou X. Proteomic based identification of manganese superoxide dismutase 2 (SOD2) as a metastasis marker for oral squamous cell carcinoma. Cancer Genomics Proteomics. 2008;5(2):85–94. [PMC free article] [PubMed] [Google Scholar]
- 43. Xu Y, Miriyala S, Fang F, Bakthavatchalu V, Noel T, Schnell DM, Wang C, St Clair WH, St Clair DK. Manganese superoxide dismutase deficiency triggers mitochondrial uncoupling and the Warburg effect [published correction appears in Oncogene. 2017;36:4087] Oncogene. 2017;36(28):4087. [DOI] [PubMed] [Google Scholar]
- 44. Kamarajugadda S, Cai Q, Chen H, Nayak S, Zhu J, He M, Jin Y, Zhang Y, Ai L, Martin SS, Tan M, Lu J. Manganese superoxide dismutase promotes anoikis resistance and tumor metastasis. Cell Death Dis. 2013;4(2):e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Loo SY, Hirpara JL, Pandey V, Tan TZ, Yap CT, Lobie PE, Thiery JP, Goh BC, Pervaiz S, Clément MV, Kumar AP. Manganese superoxide dismutase expression regulates the switch between an epithelial and a mesenchymal-like phenotype in breast carcinoma. Antioxid Redox Signal. 2016;25(6):283–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen PM, Wu TC, Shieh SH, Wu YH, Li MC, Sheu GT, Cheng YW, Chen CY, Lee H. MnSOD promotes tumor invasion via upregulation of FoxM1-MMP2 axis and related with poor survival and relapse in lung adenocarcinomas. Mol Cancer Res. 2013;11(3):261–271. [DOI] [PubMed] [Google Scholar]
- 47. Connor KM, Subbaram S, Regan KJ, Nelson KK, Mazurkiewicz JE, Bartholomew PJ, Aplin AE, Tai YT, Aguirre-Ghiso J, Flores SC, Melendez JA. Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. J Biol Chem. 2005;280(17):16916–16924. [DOI] [PubMed] [Google Scholar]
- 48. Pringle DR, Yin Z, Lee AA, Manchanda PK, Yu L, Parlow AF, Jarjoura D, La Perle KM, Kirschner LS. Thyroid-specific ablation of the Carney complex gene, PRKAR1A, results in hyperthyroidism and follicular thyroid cancer. Endocr Relat Cancer. 2012;19(3):435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miar A, Hevia D, Muñoz-Cimadevilla H, Astudillo A, Velasco J, Sainz RM, Mayo JC. Manganese superoxide dismutase (SOD2/MnSOD)/catalase and SOD2/GPx1 ratios as biomarkers for tumor progression and metastasis in prostate, colon, and lung cancer. Free Radic Biol Med. 2015;85:45–55. [DOI] [PubMed] [Google Scholar]
- 50. Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33(3):337–349. [DOI] [PubMed] [Google Scholar]
- 51. Nishida S, Akai F, Iwasaki H, Hosokawa K, Kusunoki T, Suzuki K, Taniguchi N, Hashimoto S, Tamura TT. Manganese superoxide dismutase content and localization in human thyroid tumours. J Pathol. 1993;169(3):341–345. [DOI] [PubMed] [Google Scholar]
- 52. Williams MD, Van Remmen H, Conrad CC, Huang TT, Epstein CJ, Richardson A. Increased oxidative damage is correlated to altered mitochondrial function in heterozygous manganese superoxide dismutase knockout mice. J Biol Chem. 1998;273(43):28510–28515. [DOI] [PubMed] [Google Scholar]
- 53. Oh SS, Sullivan KA, Wilkinson JE, Backus C, Hayes JM, Sakowski SA, Feldman EL. Neurodegeneration and early lethality in superoxide dismutase 2-deficient mice: a comprehensive analysis of the central and peripheral nervous systems. Neuroscience. 2012;212:201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ, Huang TT, Nelson J, Strong R, Richardson A. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16(1):29–37. [DOI] [PubMed] [Google Scholar]
- 55. Lustgarten MS, Jang YC, Liu Y, Qi W, Qin Y, Dahia PL, Shi Y, Bhattacharya A, Muller FL, Shimizu T, Shirasawa T, Richardson A, Van Remmen H. MnSOD deficiency results in elevated oxidative stress and decreased mitochondrial function but does not lead to muscle atrophy during aging. Aging Cell. 2011;10(3):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee HP, Pancholi N, Esposito L, Previll LA, Wang X, Zhu X, Smith MA, Lee HG. Early induction of oxidative stress in mouse model of Alzheimer disease with reduced mitochondrial superoxide dismutase activity. PLoS One. 2012;7(1):e28033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lark DS, Kang L, Lustig ME, Bonner JS, James FD, Neufer PD, Wasserman DH. Enhanced mitochondrial superoxide scavenging does not improve muscle insulin action in the high fat-fed mouse. PLoS One. 2015;10(5):e0126732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13(3):184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hart PC, Mao M, de Abreu AL, Ansenberger-Fricano K, Ekoue DN, Ganini D, Kajdacsy-Balla A, Diamond AM, Minshall RD, Consolaro ME, Santos JH, Bonini MG. MnSOD upregulation sustains the Warburg effect via mitochondrial ROS and AMPK-dependent signalling in cancer. Nat Commun. 2015;6(1):6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang Y, Zhang HM, Shi Y, Lustgarten M, Li Y, Qi W, Zhang BX, Van Remmen H. Loss of manganese superoxide dismutase leads to abnormal growth and signal transduction in mouse embryonic fibroblasts. Free Radic Biol Med. 2010;49(8):1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hemachandra LP, Shin DH, Dier U, Iuliano JN, Engelberth SA, Uusitalo LM, Murphy SK, Hempel N. Mitochondrial superoxide dismutase has a protumorigenic role in ovarian clear cell carcinoma. Cancer Res. 2015;75(22):4973–4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim YS, Gupta Vallur P, Phaëton R, Mythreye K, Hempel N. Insights into the Dichotomous Regulation of SOD2 in Cancer. Antioxidants. 2017;6(4):e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ansenberger-Fricano K, Ganini D, Mao M, Chatterjee S, Dallas S, Mason RP, Stadler K, Santos JH, Bonini MG. The peroxidase activity of mitochondrial superoxide dismutase. Free Radic Biol Med. 2013;54:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cen J, Zhang L, Liu F, Zhang F, Ji BS. Long-term alteration of reactive oxygen species led to multidrug resistance in MCF-7 cells. Oxid Med Cell Longev. 2016;2016:7053451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wu Y, Meitzler JL, Antony S, Juhasz A, Lu J, Jiang G, Liu H, Hollingshead M, Haines DC, Butcher D, Panter MS, Roy K, Doroshow JH. Dual oxidase 2 and pancreatic adenocarcinoma: IFN-γ-mediated dual oxidase 2 overexpression results in H2O2-induced, ERK-associated up-regulation of HIF-1α and VEGF-A. Oncotarget. 2016;7(42):68412–68433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen PM, Wu TC, Wang YC, Cheng YW, Sheu GT, Chen CY, Lee H. Activation of NF-κB by SOD2 promotes the aggressiveness of lung adenocarcinoma by modulating NKX2-1-mediated IKKβ expression. Carcinogenesis. 2013;34(11):2655–2663. [DOI] [PubMed] [Google Scholar]
- 67. Weydert C, Roling B, Liu J, Hinkhouse MM, Ritchie JM, Oberley LW, Cullen JJ. Suppression of the malignant phenotype in human pancreatic cancer cells by the overexpression of manganese superoxide dismutase. Mol Cancer Ther. 2003;2(4):361–369. [PubMed] [Google Scholar]
- 68. Zhong W, Oberley LW, Oberley TD, St Clair DK. Suppression of the malignant phenotype of human glioma cells by overexpression of manganese superoxide dismutase. Oncogene. 1997;14(4):481–490. [DOI] [PubMed] [Google Scholar]
- 69. Zhao Y, Xue Y, Oberley TD, Kiningham KK, Lin SM, Yen HC, Majima H, Hines J, St Clair D. Overexpression of manganese superoxide dismutase suppresses tumor formation by modulation of activator protein-1 signaling in a multistage skin carcinogenesis model. Cancer Res. 2001;61(16):6082–6088. [PubMed] [Google Scholar]
- 70. Chakrabarti SK, Ghosh S, Banerjee S, Mukherjee S, Chowdhury S. Oxidative stress in hypothyroid patients and the role of antioxidant supplementation. Indian J Endocrinol Metab. 2016;20(5):674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Köhrle J. Selenium and the thyroid. Curr Opin Endocrinol Diabetes Obes. 2013;20(5):441–448. [DOI] [PubMed] [Google Scholar]
- 72. Mendelsohn AR, Larrick JW. Paradoxical effects of antioxidants on cancer. Rejuvenation Res. 2014;17(3):306–311. [DOI] [PubMed] [Google Scholar]
- 73. Supabphol A, Supabphol R. Antimetastatic potential of N-acetylcysteine on human prostate cancer cells. J Med Assoc Thai. 2012;95(Suppl 12):S56–S62. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.