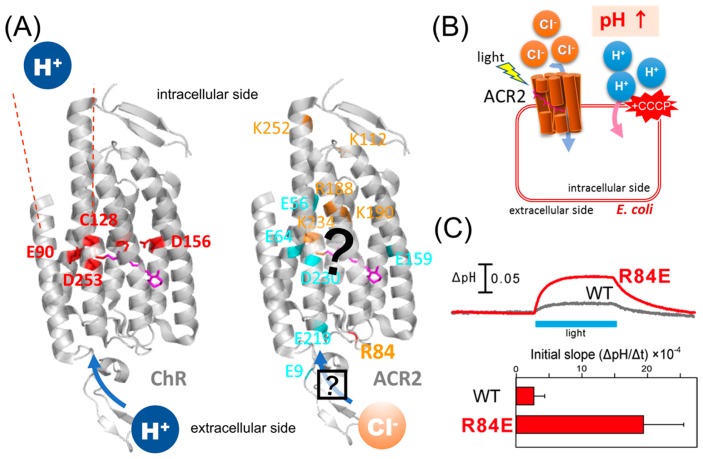
Figure 1.
Characteristics of ChRs and ACRs. (A) (Left) Crystal structure of ChR (PDB;3UG9) showing that the cation conducting pathway is formed by TM1, TM2, TM3 and TM7. Key residues for the cation transport in ChRs are colored red. (Right) High resolution structure and the anion conducting pathway of ACR2 are still unclear. Conserved basic and acidic amino acid residues are colored orange and cyan, respectively. (B) Light-induced secondary proton movement across the cell membrane by the inward chloride channeling activity of ACR2 expressed in E. coli cells, which is facilitated by the addition of CCCP. The proton movement results in increases in extracellular pH. (C) (Upper) Light-induced pH changes of E. coli cells expressing wild-type ACR2 or the R84E mutant in a solution containing 300 mM NaCl in the presence of CCCP. The cell suspensions were illuminated with blue light (480±10 nm) for 3 min (blue stripe). (Lower) Comparison of the anion transport activity of wild-type ACR2 and the R84E mutant. These data are taken from our previous study [25].