Abstract
Purpose
The aims of the study were to assess and compare grammatical deficits in written and spoken language production in subjects with agrammatic primary progressive aphasia (agPPA) and in subjects with agrammatism in the context of dominant apraxia of speech (DAOS) and to investigate neuroanatomical correlates.
Method
Eight agPPA and 21 DAOS subjects performed the picture description task of the Western Aphasia Battery (WAB) both in writing and orally. Responses were transcribed and coded for linguistic analysis. agPPA and DAOS were compared to 13 subjects with primary progressive apraxia of speech (PPAOS) who did not have agrammatism. Spearman correlations were performed between the written and spoken variables. Patterns of atrophy in each group were compared, and relationships between the different linguistic measures and integrity of Broca's area were assessed.
Results
agPPA and DAOS both showed lower mean length of utterance, fewer grammatical utterances, more nonutterances, more syntactic and semantic errors, and fewer complex utterances than PPAOS in writing and speech, as well as fewer correct verbs and nouns in speech. Only verb ratio and proportion of grammatical utterances correlated between modalities. agPPA and DAOS both showed greater involvement of Broca's area than PPAOS, and atrophy of Broca's area correlated with proportion of grammatical and ungrammatical utterances and semantic errors in writing and speech.
Conclusions
agPPA and DAOS subjects showed similar patterns of agrammatism, although subjects performed differently when speaking versus writing. Integrity of Broca's area correlates with agrammatism.
Agrammatism associated with aphasia is characterized by agrammatic or telegraphic written and spoken language production, grammatical simplification, and the omission of function words or morphemes. More specifically, these language deficits include impaired production of inflectional morphemes, lower proportions of closed class or function words, higher proportions of nouns to other open class words, use of nonfinite verb forms, errors in syntactic argument structure, and reduced sentence complexity (Ash et al., 2009; Avrutin, 2001; Grossman et al., 1996; Thompson, Ballard, Tait, Weintraub, & Mesulam, 1997; Thompson et al., 2012, 2013; Thompson & Mack, 2014; Wilson, Dronkers, et al., 2010).
Agrammatism can result from a neurodegenerative disease, in which case patients are typically diagnosed with the agrammatic variant of primary progressive aphasia (agPPA; Botha et al., 2015; Gorno-Tempini et al., 2011; Mesulam, 2003). agPPA manifests itself primarily through language deficits, leaving other cognitive functions relatively preserved. Though agrammatism is the core characteristic of this clinical syndrome, it often occurs concomitantly with apraxia of speech (AOS; Josephs et al., 2013), which is a motor speech disorder that results in decreased speech rate, articulatory groping and distortions, sound substitutions, and segmentation of syllables within multisyllabic words or across words (Duffy, 2005; Josephs et al., 2012). To meet diagnostic criteria for agPPA, language deficits and, thus, agrammatism should be the dominant clinical feature at onset and for the initial phases of the disease (Gorno-Tempini et al., 2011; Mesulam, 2003). However, patients can have agrammatism and progressive AOS where the AOS is more severe than the agrammatism (dominant AOS or DAOS; Josephs et al., 2013). Alternatively, patients can have AOS in the absence of agrammatism (primary progressive AOS or PPAOS; Josephs et al., 2012); neither DAOS nor PPAOS patients would meet criteria for agPPA given the dominance of AOS (Mesulam, 1982). Patterns of gray matter atrophy have previously been described for PPAOS (Josephs et al., 2013, 2012), agPPA, and DAOS (Josephs et al., 2013), with clear differences found between these groups supporting their clinical distinctions. The DAOS group showed patterns of atrophy similar to PPAOS with additional involvement of the inferior frontal lobe, that is, Broca's area, whereas agPPA showed widespread involvement of the language network (Josephs et al., 2013). It was, therefore, hypothesized that DAOS and agPPA may have different underlying pathologies (Josephs et al., 2013). Although agrammatism is a feature of both agPPA and DAOS, given the differing neuroanatomical associations of the two syndromes, it is possible that the specific characteristics of agrammatism will also differ. Understanding potential clinical differences between agPPA and DAOS would aid in their clinical diagnosis and aid in the separation of both syndromes from PPAOS.
A series of speech and language tests and tasks have been developed to assess language deficits in primary progressive aphasia (PPA), but few provide targeted assessments of agrammatism. Because the nature of many of these tasks relies on extralinguistic cognitive functions such as working memory and attention, as well as speech production, they may not be able to accurately represent actual language production. The most ecologically valid way to examine language abilities and deficits would be to analyze natural language production, but this has been problematic due to difficulties in quantifying linguistic structures. The biggest limitation is coding the language data to be sensitive enough to capture small deficits that are present in PPA, rather than just quantifying the frequency of different grammatical categories (Thompson et al., 2012). To date, the most comprehensive method for coding PPA language is that presented by Thompson, Shapiro, Li, and Schendel (1995), which both quantifies the frequency of different grammatical categories and captures more minute errors in syntactic argument structure that are commonly affected in the agPPA population. A few studies have performed linguistic evaluations on speech samples from patients with agPPA (Ash et al., 2010; Knibb, Woollams, Hodges, & Patterson, 2009; Wilson, Henry, et al., 2010), but none have investigated patients with DAOS or PPAOS, and therefore, it is unclear how grammatical features differ across these groups. In addition, the presence of AOS in these patients could reduce oral language output due to increased difficulty in producing words and eventual mutism. To minimize this confound, the assessment of the linguistic characteristics of written language production would be valuable. It is unclear to what degree assessments of grammatical ability from written and spoken modalities agree in this patient population.
The aim of this study was to characterize the linguistic traits of agrammatism in people with agPPA and DAOS by quantifying and comparing their written and spoken language output using a protocol similar to that reported by Thompson et al. (1995). These groups were compared to a group of PPAOS patients who, by definition, did not display agrammatism or other evidence of aphasia and thus served as a nonaphasic control group. We also assessed patterns of gray matter atrophy in the agPPA and DAOS groups compared to PPAOS to provide anatomical context to the linguistic findings. Regions in which gray matter atrophy significantly differed between agPPA and DAOS versus PPAOS were then correlated with the linguistic variables in order to better understand the biological basis for the agrammatic deficits.
Method
Subjects
Forty-two subjects were included in the study. All subjects were recruited from the Department of Neurology, Mayo Clinic, into a National Institutes of Health–funded study assessing speech and language disorders. As part of the study, each subject underwent a detailed speech-language evaluation by one of three speech-language pathologists (JRD, HMC, EAS). Clinical diagnoses were rendered by consensus after review of quantitative clinical scores and video recordings of each subject, as previously described (Josephs et al., 2012). To be included in this study, subjects received a diagnosis of agrammatic aphasia, progressive AOS, or both. Quantitative scores and video recordings of language tests were reviewed by two speech-language pathologists who made independent judgments about the presence or absence of agrammatic aphasia and AOS. The presence of agrammatism was based on function word omissions or grammatical or syntactic errors during the Western Aphasia Battery (WAB) Picture Description task, general conversation, or the narrative Writing Output subtest of the WAB Part 2. The speech-language pathologists came to consensus, using clinical judgment, on whether the AOS or aphasia was more severe. It has been demonstrated that clinical judgments regarding the relative severity of aphasia and AOS have excellent agreement with quantitative measures of severity (Josephs et al., 2013).
The agPPA group consisted of eight individuals who either presented with isolated agrammatism (n = 5) or agrammatism with concomitant AOS that was clearly less severe than the aphasia (n = 3); the DAOS group consisted of 21 subjects who presented with both agrammatism and AOS, in which the AOS was equal to or more severe than the aphasia, and the final group, PPAOS, was composed of 13 subjects who had isolated AOS and fulfilled our diagnostic criteria for PPAOS (Josephs et al., 2012). The PPAOS subjects were used as a control group because they unambiguously had no evidence of aphasia in any modality or component of language; their language performance, which is detailed below, matched those of normal controls in past studies (Bird & Franklin, 1996; Rochon, Saffran, Berndt, & Schwartz, 2000; Thompson et al., 1997).
The study was approved by the Mayo Clinic Institutional Review Board, and all patients provided informed consent under Helsinki guidelines to participate in the study.
Speech and Language Battery
All subjects underwent a thorough speech and language battery. The WAB (Kertesz, 2007) Part 1 was administered, which measures global aphasia severity and language ability through the testing of lexical content, fluency, repetition, naming, and oral language comprehension; subscores on these measures were then summed to yield an overall WAB–Aphasia Quotient (WAB-AQ). The Token Test Part V (De Renzi & Vignolo, 1962) was used to measure sentence verbal comprehension of various syntactic structures, and the 15-item Boston Naming Test (Lansing, Ivnik, Cullum, & Randolph, 1999) measured subjects' confrontation-naming. Speech production tasks that included word and sentence repetitions, vowel prolongation, speech alternating motion rates (e.g., rapid repetition of “puh-puh-puh…”), and speech sequential motion rates (e.g., rapid repetition of “puh-tuh-kuh…”), as well as the spoken language tasks within the WAB and conversational speech samples, were used to assess motor speech abilities. An adapted version of the Apraxia of Speech Rating Scale (Duffy, Strand, & Josephs, 2014) and the Motor Speech Disorders Scale (Yorkston, Strand, Miller, Hillel, & Smith, 1993) were used to measure AOS severity and associated functional impairment, respectively. Finally, subjects were tested on the short form of the Northwestern Anagram Test (Weintraub et al., 2009), which aims to assess written syntactic performance.
Linguistic Analysis
Subjects were video-recorded as they described the WAB picnic scene. Instructions encouraged the subjects to “speak in sentences.” The same task was completed with written description as one component of the WAB Supplemental Reading and Writing battery. The spoken and written picture description language samples were transcribed and coded in CHAT transcription format for use in the Computerized Language Analysis software (MacWhinney, 2000). The transcriptions included word-level codes for each grammatical category (e.g., nouns, verbs, and/or articles) as well as codes for each inflectional and derivational morpheme. Word-level error codes (e.g., semantic errors, argument structure errors, and/or verb morphology errors) and utterance-level codes (e.g., whether the utterance was grammatical, ungrammatical [one to three errors, including the aforementioned word-level errors or missing words], or a nonutterance [utterances with nonfinite matrix verbs or isolated noun phrase]) were also marked; these coded variables are summarized in Table 1.
Table 1.
Linguistic variables measured.
| Measure | Description |
|---|---|
| Mean length of utterance | Number of total morphemes divided by number of utterances. |
| Word count | Total number of words produced. |
| Verb ratio | Number of verbs divided by total number of words. |
| Noun ratio | Number of nouns divided by total number of words. |
| Function word ratio | Number of closed class words (articles, pronouns, prepositions) divided by total number of words. |
| Correct verb ratio | Number of correctly produced verbs divided by total number of verbs. |
| Grammatical utterance ratio | Number of grammatical utterances produced divided by total number of utterances. |
| Ungrammatical utterance ratio | Number of ungrammatical utterances produced divided by total number of utterances. Ungrammatical utterance had at least one but no more than three errors; these errors could involve a missing word, an incorrect word, or words in the wrong order. |
| Nonutterance ratio | Number of nonutterances divided by total number of utterances. These would include noun phrases and any utterance in which a verb was not produced. |
| Semantic error ratio | Number of semantic errors over total number of words. This was noted if the wrong word was produced. |
| Syntactic error ratio | Number of syntactic errors over total number of words. Syntactic errors included mistakes in verb morphology or producing words in the wrong order. |
| Complex utterance ratio | Number of syntactically complex utterances divided by total number of utterances. Utterances were considered syntactically complex if there was embedding/subordination. |
Author KAT, who had extensive experience with linguistic transcriptions, transcribed these samples; 20% of the transcriptions were then repeated by author RLU, and interrater reliability was tested by calculating Cohen's kappa coefficients. Kappa values ranged between .68 and .81, indicating substantial to almost perfect agreement for all variables (Landis & Koch, 1977). Moreover, there was 98% agreement for lexical items and 96% agreement for utterance boundaries between the two raters. Seven utterances from the DAOS subjects (totaling 4% of DAOS and 1.7% of all utterances) were excluded because they were unintelligible.
The Computerized Language Analysis software (MacWhinney, 2000) was used to calculate the number of utterances, word count, mean length of utterance (MLU), verb count, noun count, function word count (determiners, pronouns, and prepositions), number of semantic and syntactic errors, the number of complex utterances (utterances that had embedding), and the number of each utterance type (grammatical, ungrammatical, non-utterance; see Table 1). Ratios of these variables were calculated to minimize influence of differences in number of utterances and total number of words produced. As agrammatism has been characterized by telegraphic speech and writing, grammatical simplification, and the omission of function words, among other features, the specific variables used in this study were chosen and quantified in this way because they reflect these deficits. Agrammatic subjects can be expected to show reduced sentence length and complexity, as measured by the MLU and the ratio of complex utterances; fewer function words, as measured by the ratio of function words to total words; and verbal and syntactic difficulties, as measured by the ratio of syntactic errors and overall sentence grammaticality. In addition, increased production of nouns in relation to other words and the use of nonutterances, which lack a tensed verb, reflect the use of telegraphic speech and writing.
Magnetic Resonance Imaging Analysis
All subjects in the study underwent a 3T volumetric head magnetic resonance imaging within 1 day of the clinical assessments, as previously described (Josephs et al., 2012). Voxel-based morphometry was used to perform voxel-level comparisons of gray matter volume between groups. All magnetic resonance imaging scans were normalized to a customized template and segmented into gray matter, white matter, and cerebrospinal fluid using unified segmentation (Ashburner & Friston, 2005). The gray matter images were modulated and smoothed with an 8-mm full-width-at-half maximum smoothing kernel. t Tests were used to compare voxel-level gray matter volumes between agPPA and PPAOS and to compare DAOS and PPAOS, with results shown at p < .001, uncorrected for multiple comparisons, with a cluster threshold of 200 voxels.
The total gray matter volume of Broca's area was also calculated for each patient in order to allow correlations between Broca's area volume and the spoken and written linguistic data (statistical methods described below). Atlas-based parcellation using the automated anatomical labeling atlas (Tzourio-Mazoyer et al., 2002) was used to calculate gray matter volumes of the left inferior triangularis and the left inferior opercularis for each subject. The gray matter volumes from these two regions were summed in each subject to produce a total Broca's area volume. These volumes were divided by total intracranial volume to correct for head size.
Statistics
The linguistic variables were compared independently for writing and speech samples between the three subject groups, agPPA, DAOS, and PPAOS, using a nonparametric pairwise Wilcoxon rank sum comparison test in RStudio, with all p values being corrected for multiple comparisons using the false discovery rate correction. Spearman rank correlations were used to correlate performance on the writing and speech scores for each linguistic variable; these correlations were performed using all cohorts combined and within only the agPPA and DAOS cohorts (i.e., excluding the PPAOS subjects).
The total intracranial volume-corrected volumes of Broca's area were converted to age-corrected z scores representing the degree of abnormality compared to a healthy control cohort (n = 75, 40 female, mean age = 71, range 51–89). We fitted linear regression using age as a predictor and mean gray matter volume as an outcome. We then extracted the intercept (beta0), slope (beta1), and residual standard error (sigma) from the model. The age-adjusted z score was calculated as follows: (mean volume – (beta0 + beta1 * age)) / sigma. The age-adjusted z scores were correlated with the speech and writing linguistic variables using Spearman rank correlations, which also were subject to false discovery rate correction. These correlations were performed using all cohorts combined (i.e., agPPA, DAOS, and PPAOS) and within only the agPPA and DAOS cohorts (i.e., excluding the PPAOS subjects).
Results
Group Comparisons
There were no significant differences between diagnostic groups regarding age or gender (see Table 2). The agPPA and DAOS groups did not differ on the WAB-AQ or Northwestern Anagram Test, but both of these cohorts performed significantly worse than the PPAOS cohort on these measures; agPPA subjects performed worse than DAOS subjects on the Token Test, and both of these groups' performance was worse than that of the PPAOS subjects. The DAOS and PPAOS groups had significantly lower scores than agPPA on the Apraxia of Speech Rating Scale, reflecting the severity of the AOS.
Table 2.
Demographics and clinical variables.
| Variable | agPPA (n = 8) | DAOS (n = 21) | PPAOS (n = 13) | False discovery rate p value |
||
|---|---|---|---|---|---|---|
| agPPA–DAOS | agPPA–PPAOS | DAOS–PPAOS | ||||
| Age at exam | 68 (62–74) | 65 (61–72) | 75 (61–77) | p = .92 | p = .40 | p = .45 |
| Gender (female, male) | 5, 3 | 12, 9 | 4, 9 | p = .65 | p = .18 | p = .23 |
| WAB-AQ (/100) | 86 (79–90) | 88 (84–94) | 98 (97–99) | p = .21 | p = .0003* | p < .0001* |
| NAT (/10) | 6 (6–8) | 6 (5–8) | 9 (9–10) | p = .69 | p = .004* | p = .0006* |
| BNT (/15) | 14 (11–14) | 13 (11–14) | 15 (14–15) | p = .9 | p = .07 | p = .01* |
| Token Test (/22) | 13 (6–16) | 19 (15–20) | 20 (20–22) | p = .01* | p = .0002* | p = .0004* |
| MSD (/10) | 9 (7–10) | 6 (5–6) | 7 (6–7) | p = .01* | p = .07 | p = .03* |
| ASRS (/64) | 4 (1–9) | 24 (21–31) | 19 (17–25) | p < .0001* | p = .0002* | p = .14 |
Note. Data shown as median (interquartile range) or number of participants. * indicates significance after false discovery rate correction. WAB-AQ = Western Aphasia Battery–Aphasia Quotient; NAT = Northwestern Anagram Test; BNT = Boston Naming Test; MSD = Motor Speech Disorder Scale; ASRS = Apraxia of Speech Rating Scale; agPPA = agrammatic primary progressive aphasia; DAOS = dominant apraxia of speech; PPAOS = primary progressive apraxia of speech.
In the writing samples, agPPA and DAOS did not perform differently on any of the linguistic measures (see Table 3). However, PPAOS subjects produced significantly higher MLUs, more grammatical utterances, fewer nonutterances, fewer syntactic and semantic errors, and more complex utterances than both agPPA and DAOS. Furthermore, PPAOS subjects produced significantly more function words than the agPPA subjects. Similar results were observed in the speech samples. The agPPA and DAOS groups performed similarly, whereas PPAOS subjects produced higher MLUs, more grammatical and fewer nonutterances, fewer syntactic and semantic errors, and more complex sentences (see Table 4). In addition, the agPPA and DAOS groups had a higher proportion of nouns and fewer correct verbs than PPAOS. The only difference between the agPPA and DAOS groups was that the former produced more semantic errors (see Table 4).
Table 3.
Writing variable group comparisons.
| Measure | agPPA | DAOS | PPAOS |
p value |
||
|---|---|---|---|---|---|---|
| agPPA–DAOS | agPPA–PPAOS | DAOS–PPAOS | ||||
| Mean length of utterance | 5.57 (4.00–6.25) | 8.83 (7.13–9.80) | 11.00 (9.57–13.50) | p = .16 | p = .01* | p = .02* |
| Word count | 34 (20.5–46) | 42 (39–49) | 49 (38–57) | p = .23 | p = .20 | p = .51 |
| Verb ratio | 18.30 (13.59–21.25) | 14.29 (12.50–18.00) | 14.29 (13.79–15.38) | p = .22 | p = .20 | p = .66 |
| Noun ratio | 33.53 (31.13–39.77) | 31.25 (30.43–34.15) | 30.61 (29.17–32.00) | p = .29 | p = .10 | p = .11 |
| Function word ratio | 41.33 (21.21–44.24) | 45.45 (31.24–48.48) | 47.62 (46.51–50.00) | p = .62 | p = .02* | p = .05 |
| Correct verb ratio | 81.94 (48.21–100) | 85.71 (71.43–1.00) | 100 (87.50–100) | p = .63 | p = .09 | p = .09 |
| Grammatical utterance ratio | 16.67 (0.00–58.33) | 40.00 (25.00–60.00) | 85.71 (83.33–1.00) | p = .33 | p = .003* | p < .0001* |
| Ungrammatical utterance ratio | 25.00 (10.71–59.52) | 37.50 (28.57–50.00) | 0.00 (0.00–0.17) | p = .45 | p = .12 | p = .0002* |
| Nonutterance ratio | 31.43 (0.00–54.17) | 0.00 (0.00–28.57) | 0.00 (0.00–0.00) | p = .21 | p = .01* | p = .01* |
| Semantic error ratio | 2.27 (0.00–7.63) | 1.52 (0.00–2.44) | 0.00 (0.00–0.00) | p = .43 | p = .01* | p = .001* |
| Syntactic error ratio | 3.94 (1.07–6.04) | 2.08 (0.00–6.06) | 0.00 (0.00–1.75) | p = .6 | p = .03* | p = .04* |
| Complex utterance ratio | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 14.29 (0.00–25.00) | p = .21 | p = .02* | p = .03* |
Note. Data shown as median (interquartile range). * indicates significance after false discovery rate correction. agPPA = agrammatic primary progressive aphasia; DAOS = dominant apraxia of speech; PPAOS = primary progressive apraxia of speech.
Table 4.
Speech variable group comparisons.
| Measure | agPPA | DAOS | PPAOS |
p value |
||
|---|---|---|---|---|---|---|
| agPPA–DAOS | agPPA–PPAOS | DAOS–PPAOS | ||||
| Mean length of utterance | 7.29 (6.44–8.26) | 7.83 (5.76–10.83) | 9.91 (9.63–11.64) | p = .77 | p = .009* | p = .01* |
| Word count | 70 (44–84.75) | 54 (34–88) | 84 (74–95) | p = .56 | p = .17 | p = .03* |
| Verb ratio | 16.58 (15.06–18.18) | 15.79 (13.76–17.06) | 15.79 (14.91–16.67) | p = .44 | p = .49 | p = 1.0 |
| Noun ratio | 31.99 (28.23–37.27) | 29.97 (26.43–38.05) | 26.32 (24.59–28.79) | p = .75 | p = .04* | p = .04* |
| Function word ratio | 42.42 (38.77–44.01) | 42.70 (0.00–46.97) | 47.52 (42.86–49.46) | p = .94 | p = .14 | p = .15 |
| Correct verb ratio | 83.97 (70.83–89.39) | 75.00 (61.25–93.73) | 100 (100–100) | p = .87 | p = .0004* | p = .0002* |
| Grammatical utterance ratio | 45.63 (24.55–61.63) | 40.00 (29.67–59.03) | 87.50 (85.71–91.67) | p = .81 | p = .002* | p < .0001* |
| Ungrammatical utterance ratio | 56.25 (19.79–73.02) | 30.77 (25.00–50.00) | 10.00 (0.00–14.29) | p = .47 | p = .006* | p < .0001* |
| Nonutterance ratio | 7.14 (0.00–22.92) | 16.67 (0.00–39.23) | 0.00 (0.00–0.00) | p = .3 | p = .02* | p = .002* |
| Semantic error ratio | 3.57 (2.29–5.78) | 0.00 (0.00–1.42) | 0.00 (0.00–0.00) | p = .005* | p = .0001* | p = .01* |
| Syntactic error ratio | 2.44 (2.16–4.77) | 0.00 (0.00–1.42) | 0.00 (0.00–0.00) | p = .89 | p = .0004* | p = .0002* |
| Complex utterance ratio | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 12.50 (9.09–28.57) | p = .18 | p = .002* | p = .0007* |
Note. Data shown as median (interquartile range). * indicates significance after false discovery rate correction. agPPA = agrammatic primary progressive aphasia; DAOS = dominant apraxia of speech; PPAOS = primary progressive apraxia of speech.
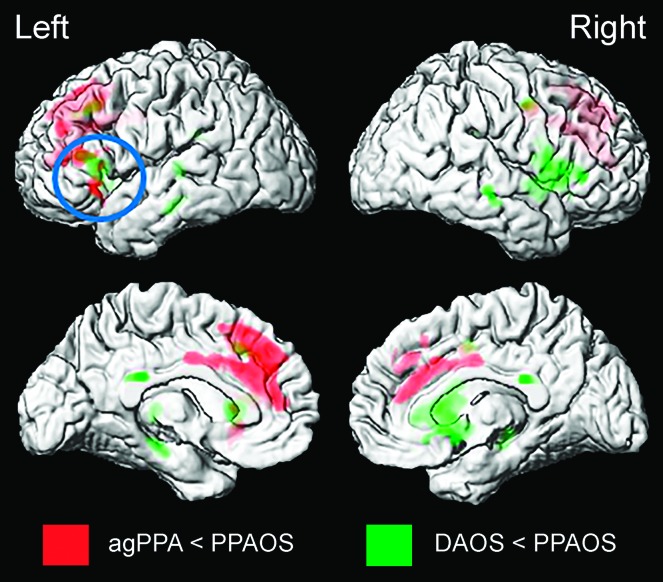
Both agPPA and DAOS groups showed greater volume loss in Broca's area compared to PPAOS in the voxel-level comparisons (see Figure 1). The agPPA group also showed greater volume loss in the medial prefrontal cortex, and the DAOS group also showed greater volume loss in the right inferior frontal lobe compared to PPAOS.
Figure 1.
Patterns of gray matter atrophy in agrammatic primary progressive aphasia (agPPA; red) and dominant apraxia of speech (DAOS; green) compared to primary progressive apraxia of speech (PPAOS). Results are shown on three-dimensional renders of the brain at p < .001, with a cluster threshold of 200 voxels.
Correlation Analyses
Significant correlations between measures derived from the writing and speech samples for the entire cohort were observed for correct verb ratio, proportion of grammatical utterances, proportion of ungrammatical utterances, semantic errors and syntactic errors, with the strongest correlation observed for proportion of grammatical utterances (r = .97; see Table 5). When considering only the agPPA and DAOS patients, correlations between speech and writing samples were observed only for the proportion of correct verbs and proportion of grammatical utterances; the other correlations were not significant without the PPAOS subjects (see Table 5).
Table 5.
Correlations in linguistic scores from writing versus speech samples.
| Measure | Estimated false discovery rate p value |
|
|---|---|---|
| All subjects | agPPA & DAOS | |
| Mean length of utterance | r = .34, p = .06 | r = .24, p = .63 |
| Word count | r = .13, p = .45 | r = .18, p = .64 |
| Verb ratio | r = −.12, p = .45 | r = −.15, p = .64 |
| Noun ratio | r = .33, p = .06 | r = .18, p = .64 |
| Function word ratio | r = .13, p = .50 | r = .03, p = .94 |
| Correct verb ratio | r = .55, p = .002* | r = .55, p = .02* |
| Grammatical utterance ratio | r = .97, p < .001* | r = .93, p < .0001* |
| Ungrammatical utterance ratio | r = .52, p = .003* | r = .02, p = .94 |
| Nonutterance ratio | r = .29, p = .10 | r = .12, p = .64 |
| Semantic error ratio | r = .45, p = .01* | r = .32, p = .35 |
| Syntactic error ratio | r = .50, p = .004* | r = .40, p = .18 |
| Complex utterance ratio | r = .22, p = .22 | r = −.15, p = .64 |
Note. * indicates significance after false discovery rate correction. agPPA = agrammatic primary progressive aphasia; DAOS = dominant apraxia of speech.
Because Broca's area was significantly more affected in the agPPA and DAOS groups compared to PPAOS, volumes of this structure were then correlated with the linguistic speech and writing variables, as well as the WAB-AQ scores. A significant positive correlation was found between Broca's area volume and the ratio of grammatical utterances, whereas significant negative correlations were found regarding the ratio of ungrammatical utterances and the ratio of semantic errors, for both spoken and written language production. The noun and function word ratios from the written language samples also were significantly related to volume of Broca's area. Excluding PPAOS from these correlations, only the proportion of correctly produced verbs in the written sample negatively correlated with gray matter volume in Broca's area (see Table 6).
Table 6.
Correlations in linguistic scores from writing and speech samples versus Broca's area volume.
| Measure | Estimated false discovery rate p value |
|||
|---|---|---|---|---|
| Writing |
Speech |
|||
| All subjects | agPPA & DAOS | All subjects | agPPA & DAOS | |
| Mean length of utterance | r = .17, p = .28 | r = −.04, p = .85 | r = .28, p = .08 | r = .25, p = .22 |
| Word count | r = −.1, p = .55 | r = −.18, p = .35 | r = .12, p = .48 | r = .13, p = .52 |
| Verb ratio | r = .01, p = .93 | r = .04, p = .83 | r = .02, p = .89 | r = .02, p = .92 |
| Noun ratio | r = −.34, p = .03* | r = −.29, p = .13 | r = −.12, p = .46 | r = .02, p = .93 |
| Function word ratio | r = .3, p < .05* | r = .12, p = .54 | r = −.02, p = .9 | r = −.13, p = .54 |
| Correct verb ratio | r = −.08, p = .61 | r = −.37, p < .05* | r = .28, p = .09 | r = −.07, p = .75 |
| Grammatical utterance ratio | r = .35, p = .03* | r = .01, p = .94 | r = .38, p = .02* | r = .07, p = .73 |
| Ungrammatical utterance ratio | r = −.36, p = .02* | r = −.14, p = .47 | r = −.45, p < .0001* | r = −.17, p = .39 |
| Nonutterance ratio | r = −.18, p = .26 | r = .09, p = .65 | r = −.07, p = .67 | r = .2, p = .32 |
| Semantic error ratio | r = −.35, p = .02* | r = −.17, p = .38 | r = −.39, p = .01* | r = −.25, p = .21 |
| Syntactic error ratio | r = −.02, p = .88 | r = .26, p = .18 | r = −.27, p = .1 | r = .1, p = .62 |
| Complex utterance ratio | r = .24, p = .13 | r = .19, p = .34 | r = .21, p = .21 | r = −.15, p = .47 |
Note. * indicates significance after false discovery rate correction. agPPA = agrammatic primary progressive aphasia; DAOS = dominant apraxia of speech.
Discussion
Our results demonstrate that agPPA and DAOS show similar linguistic profiles of agrammatism, which are distinct from those observed in PPAOS. We also demonstrate that agrammatic errors were greater in the spoken rather than written modality. In past studies that investigated language difficulties in patients with agrammatism, subjects with dominant agrammatism and those with dominant AOS were combined together to form one experimental group. In this study, however, we directly compared these two groups and found that there are virtually no group differences between agPPA and DAOS subjects regarding their linguistic production measures. The only variable in which they were different was in semantic errors in the speech sample, with the agPPA group performing worse than the DAOS group. Given that making semantic errors is not a defining characteristic of agrammatism, it is unclear at this time why this difference was observed; the fact that it was only present in the speech and not the writing samples may be due to extralinguistic pressures that associated with speaking but not writing, which are discussed below.
As expected, the two agrammatic groups differed significantly from the PPAOS subjects' language production, specifically showing decreased MLU, fewer grammatical utterances, more isolated noun phrases, more syntactic and semantic errors, and fewer complex utterances, mirroring the results of past studies interested in language production in agPPA (Ash et al., 2010; Graham, Patterson, & Hodges, 2004; Knibb et al., 2009; Saffran, Berndt, & Schwartz, 1989; Thompson et al., 2012; Thompson & Mack, 2014; Wilson, Henry, et al., 2010). The performance of the PPAOS subjects for MLU, noun ratio, grammatical utterance ratio, and function word ratio, all match the previously reported values of normal controls (Bird & Franklin, 1996; Rochon et al., 2000; Thompson et al., 1997), supporting the clinical judgment of intact grammar and general lack of aphasia in PPAOS. Though some past researchers have documented that agrammatism results in a lower proportion of function words in language production (Thompson et al., 1997; Thompson & Mack, 2014, among others), others have not found this to be the case (Graham et al., 2004). We only observed a lower proportion of function words in agPPA in the writing samples.
Previous linguistic analyses of agrammatism in agPPA have focused on assessing spoken language samples. One previous study assessed both written and spoken language production in patients with progressive nonfluent aphasia (Graham et al., 2004). They found similar patterns of linguistic abnormalities in both the writing and speech samples in progressive nonfluent aphasia compared to controls. One notable exception, however, was that the rate of grammatical errors were only abnormal in the written sample. This difference was attributed to the fact that some patients wrote telegraphically because they were unable to write in full sentences or because they forgot the instructions to do so (Graham et al., 2004). However, the patients in that study did not show much evidence of agrammatism in their speech or writing (Graham et al., 2004) and thus may not be comparable to the patients in our agPPA and DAOS groups. In addition, that study did not perform correlations between the speech and writing variables. In our cohorts, overall, the agrammatic subjects made more errors in the speech samples than in the writing samples, thus leading to more significant differences between the agrammatic groups and the PPAOS group in the spoken modality. This is consistent with one study that showed greater impairment of verb naming in spoken than written production, in three cases of progressive nonfluent aphasia (Hillis, Tuffiash, & Caramazza, 2002). It is therefore possible that assessments of agrammatism in speech samples may be more sensitive than the assessment of writing samples.
Our findings likely reflect a combination of factors. When writing, there is time to plan out what is going to be written, so there is less of a time constraint in accessing the lexicon and in preparing the structure of the phrase; these errors may be caught and corrected in this planning time. In contrast, the timely pressure of wanting to speak quickly and fluidly may force more errors that cannot be reversed when speaking. Because of this, the time allotted to plan which words will be used and the syntactic structure of speech is significantly less in spoken language. In addition, more errors in the speech samples could be attributed to language anxiety, which negatively affects spoken language performance. Language anxiety consists of excessive self-evaluation, worry about potential errors, and apprehension about the opinions of others; these thoughts can distract the patient's attention from the task at hand, dividing his or her cognitive resources (Eysenck, 1979). This decreases the efficiency of an individual's cognitive performance, resulting in slower or impaired lexical retrieval, potentially explaining some of our results, such as the increased semantic and syntactic errors in speech versus writing samples.
Despite the observation that there were more errors overall in spoken versus written language production, performance on the two modalities were correlated for MLU, ratios of correct verbs, semantic errors, syntactic errors, grammatical utterances, and ungrammatical utterances. The correlation for the ratio of grammatical utterances was particularly strong. The fact that these variables were so strongly correlated between speech and writing demonstrates that the linguistic deficits associated with agrammatism are not restricted to one modality of language production: If someone's grammar is impaired, the impairment will negatively impact all language production in spoken and written expression. When the PPAOS subjects were removed from these correlations and the agPPA and DAOS subjects remained, only the ratio of correctly produced verbs and the proportion of grammatical utterances were correlated between speech and writing. The fact that correlations were not observed for the other variables could reflect the aforementioned observation that speech samples seemed more impaired than the written samples and the notion that speech may be negatively impacted by difficulties with nonlinguistic cognitive abilities, such as executive dysfunction, and by social, nonlinguistic factors, such as language anxiety, which could be particularly problematic in the agrammatic patients. In writing, as opposed to speech, there seem to be fewer extralinguistic variables that could contaminate the language production, and for this reason, written language performance may be a better way to assess linguistic ability within this agrammatic population. This is supported by the fact that Broca's area volume correlated more strongly with written rather than spoken measures in the agrammatic patients. However, it is also possible that we lacked statistical power to detect correlations in the smaller cohort of only agPPA and DAOS patients.
The previously discussed findings confirm that the linguistic errors investigated, such as reduced MLU, higher proportion of nouns, and semantic and syntactic errors, are markers of agrammatism. Following this, these variables were then correlated with volume of Broca's area. We found some evidence that Broca's area was more affected in the agPPA and DAOS groups compared to PPAOS in the voxel-wise group comparisons, although the results did not survive a correction for multiple comparisons. Despite this, across all our cohorts, the ratio of nouns, function words, grammatical and ungrammatical utterances, and semantic errors were all correlated with gray matter volume of Broca's area after correction for multiple comparisons. When PPAOS subjects were eliminated from the analyses, these correlations disappeared, suggesting that their relatively less affected Broca's area and near-normal linguistic performance may have been contributing to these correlations. Nonetheless, when subjects with agrammatism were isolated, their Broca's area gray matter volume negatively correlated with their ability to correctly produce verbs in their writing samples. This was surprising because it was expected that a lower proportion of correctly produced verbs would be associated with a smaller Broca's area volume (i.e., a positive association). Further qualitative inspection of the writing samples from the agPPA and DAOS cohorts was performed, and two possible explanations for this finding were revealed. The subjects with agrammatism produced a large proportion of utterances that lacked a verb entirely. Thus, some of the severely impaired subjects with agrammatism produced just isolated noun phrases, so their writing samples would not reflect a deficit in correct verbal usage because no verbs were used. The correct verb ratio variable did not finely capture this aspect of the impaired language production. The nature of the verbs produced may also have contributed to this finding. Many of the verbs produced by the agrammatic subjects in the writing samples were syntactically simple; there was a tendency to produce intransitive verbs (i.e., verbs that have just one argument as opposed to two or even three). Intransitive verbs are syntactically less complex and, thus, may be easier for the agrammatic patients to produce correctly. By limiting their verb selection to intransitives, they are able to achieve more grammatical utterances, which could have led to this negative correlation, despite a possible deficit with more complex verbs.
Generally, more linguistic variables from the writing samples correlated with Broca's area than the variables from the speech samples, perhaps suggesting a more robust association between writing measures and anatomy of Broca's area. Although there is strong evidence that syntactic performance is dependent on many regions in the language network (Grodzinsky, 2000; Papathanassiou et al., 2000; Stowe, Haverkort, & Zwarts, 2005), the present results nonetheless support the notion that Broca's area plays a role in this aspect of language (Amici et al., 2007; Geschwind, 1970; Gorno-Tempini et al., 2004; Grossman et al., 2013; Josephs et al., 2013, 2006; Rohrer et al., 2009; Whitwell et al., 2017, 2013), as subjects whose brains were unaffected in this region did not produce agrammatic deficits.
In this study, we have shown that there are many measurable linguistic factors that can distinguish the speech of patients with agrammatism from those without, but there is no linguistic distinction within subjects showing agrammatism between those with dominant AOS and those for whom the agrammatism is dominant. Analyzing agrammatism in speech appears to be more sensitive than assessing agrammatism in writing in this cohort. We have also shown that some of the measured variables correlate well with the amount of gray matter degeneration in Broca's area, a region of the language network that significantly differed between patients with and without diagnosed agrammatism.
Acknowledgments
This research was supported by National Institutes of Health Grant R01-DC010367 awarded to Keith A. Josephs and Grant R01-DC12519 awarded to Jennifer L. Whitwell.
Funding Statement
This research was supported by National Institutes of Health Grant R01-DC010367 awarded to Keith A. Josephs and Grant R01-DC12519 awarded to Jennifer L. Whitwell.
References
- Amici S., Brambati S. M., Wilkins D. P., Ogar J., Dronkers N. L., Miller B. L., & Gorno-Tempini M. L. (2007). Anatomical correlates of sentence comprehension and verbal working memory in neurodegenerative disease. Journal of Neuroscience, 27(23), 6282–6290. https://doi.org/10.1523/JNEUROSCI.1331-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S., McMillan C., Gunawardena D., Avants B., Morgan B., Khan A., … Grossman M. (2010). Speech errors in progressive non-fluent aphasia. Brain and Language, 113(1), 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S., Moore P., Vesely L., Gunawardena D., McMillan C., Anderson C., … Grossman M. (2009). Non-fluent speech in frontotemporal lobar degeneration. Journal of Neurolinguistics, 22(4), 370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J., & Friston K. J. (2005). Unified segmentation. NeuroImage, 26(3), 839–851. [DOI] [PubMed] [Google Scholar]
- Avrutin S. (2001). Linguistics and agrammatism. Glot International, 5(3), 1–11. [Google Scholar]
- Bird H., & Franklin S. (1996). Cinderella revisited: A comparison of fluent and non-fluent aphasic speech. Journal of Neurolinguistics, 9(3), 187–206. [Google Scholar]
- Botha H., Duffy J. R., Whitwell J. L., Strand E. A., Machulda M. M., Schwarz C. G., … Josephs K. A. (2015). Classification and clinicoradiologic features of primary progressive aphasia (PPA) and apraxia of speech. Cortex, 69, 220–236. https://doi.org/10.1016/j.cortex.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Renzi A., & Vignolo L. A. (1962). Token Test: A sensitive test to detect receptive disturbances in aphasics. Brain: A Journal of Neurology, 85, 665–678. [DOI] [PubMed] [Google Scholar]
- Duffy J. R. (2005). Motor speech disorders: Substrates, differential diagnosis, and management. St. Louis, MO: Elsevier Mosby. [Google Scholar]
- Duffy J. R., Strand E. A., & Josephs K. A. (2014). Motor speech disorders associated with primary progressive aphasia. Aphasiology, 28(8–9), 1004–1017. https://doi.org/10.1080/02687038.2013.869307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck M. W. (1979). Anxiety, learning, and memory: A reconceptualization. Journal of Research in Personality, 13(4), 363–385. [Google Scholar]
- Geschwind N. (1970). The organization of language and the brain. Science, 170(3961), 940–944. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini M. L., Dronkers N. F., Rankin K. P., Ogar J. M., Phengrasamy L., Rosen H. J., … Miller B. L. (2004). Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology, 55(3), 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M. L., Hillis A. E., Weintraub S., Kertesz A., Mendez M., Cappa S. F., … Grossman M. (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. https://doi.org/10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham N. L., Patterson K., & Hodges J. R. (2004). When more yields less: Speaking and writing deficits in nonfluent progressive aphasia. Neurocase, 10(2), 141–155. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y. (2000). The neurology of syntax: Language use without Broca's area. Behavioral and Brain Sciences, 23(1), 1–21. [DOI] [PubMed] [Google Scholar]
- Grossman M., Mickanin J., Onishi K., Hughes E., D'Esposito M., Ding X. S., … Reivich M. (1996). Progressive nonfluent aphasia: Language, cognitive, and PET measures contrasted with probable Alzheimer's disease. Journal of Cognitive Neuroscience, 8(2), 135–154. https://doi.org/10.1162/jocn.1996.8.2.135 [DOI] [PubMed] [Google Scholar]
- Grossman M., Powers J., Ash S., McMillan C., Burkholder L., Irwin D., & Trojanowski J. Q. (2013). Disruption of large-scale neural networks in non-fluent/agrammatic variant primary progressive aphasia associated with frontotemporal degeneration pathology. Brain and Language, 127(2), 106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis A. E., Tuffiash E., & Caramazza A. (2002). Modality-specific deterioration in naming verbs in nonfluent primary progressive aphasia. Journal of Cognitive Neuroscience, 14(7), 1099–1108. https://doi.org/10.1162/089892902320474544 [DOI] [PubMed] [Google Scholar]
- Josephs K. A., Duffy J. R., Strand E. A., Machulda M. M., Senjem M. L., Lowe V. J., … Whitwell J. L. (2013). Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology, 81(4), 337–345. https://doi.org/10.1212/WNL.0b013e31829c5ed5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs K. A., Duffy J. R., Strand E. A., Machulda M. M., Senjem M. L., Master A. V., … Whitwell J. L. (2012). Characterizing a neurodegenerative syndrome: Primary progressive apraxia of speech. Brain, 135(Pt. 5), 1522–1536. https://doi.org/10.1093/brain/aws032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs K. A., Duffy J. R., Strand E. A., Whitwell J. L., Layton K. F., Parisi J. E., … Petersen R. C. (2006). Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain, 129(Pt. 6), 1385–1398. https://doi.org/10.1093/brain/awl078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A. (2007). Western Aphasia Battery–Revised. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Knibb J. A., Woollams A. M., Hodges J. R., & Patterson K. (2009). Making sense of progressive non-fluent aphasia: An analysis of conversational speech. Brain, 132(10), 2734–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis J. R., & Koch G. G. (1977). The measurement of observer agreement for categorical data. Biometrics, 33, 159–174. [PubMed] [Google Scholar]
- Lansing A. E., Ivnik R. J., Cullum C. M., & Randolph C. (1999). An empirically derived short form of the Boston Naming Test. Archives of Clinical Neuropsychology, 14(6), 481–487. [PubMed] [Google Scholar]
- MacWhinney B. (2000). The CHILDES project: The database (Vol. 2). Mahwah, NJ: Lawrence Erlbaum. [Google Scholar]
- Mesulam M. M. (1982). Slowly progressive aphasia without generalized dementia. Annals of Neurology, 11(6), 592–598. [DOI] [PubMed] [Google Scholar]
- Mesulam M. M. (2003). Primary progressive aphasia—A language-based dementia. New England Journal of Medicine, 349(16), 1535–1542. [DOI] [PubMed] [Google Scholar]
- Papathanassiou D., Etard O., Mellet E., Zago L., Mazoyer B., & Tzourio-Mazoyer N. (2000). A common language network for comprehension and production: A contribution to the definition of language epicenters with PET. NeuroImage, 11(4), 347–357. [DOI] [PubMed] [Google Scholar]
- Rochon E., Saffran E. M., Berndt R. S., & Schwartz M. F. (2000). Quantitative analysis of aphasic sentence production: Further development and new data. Brain and Language, 72(3), 193–218. [DOI] [PubMed] [Google Scholar]
- Rohrer J., Warren J., Modat M., Ridgway G., Douiri A., Rossor M., … Fox N. C. (2009). Patterns of cortical thinning in the language variants of frontotemporal lobar degeneration. Neurology, 72(18), 1562–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffran E. M., Berndt R. S., & Schwartz M. F. (1989). The quantitative analysis of agrammatic production: Procedure and data. Brain and Language, 37(3), 440–479. [DOI] [PubMed] [Google Scholar]
- Stowe L. A., Haverkort M., & Zwarts F. (2005). Rethinking the neurological basis of language. Lingua, 115(7), 997–1042. [Google Scholar]
- Thompson C. K., Ballard K. J., Tait M. E., Weintraub S., & Mesulam M. M. (1997). Patterns of language decline in non-fluent primary progressive aphasia. Aphasiology, 11(4–5), 297–321. [Google Scholar]
- Thompson C. K., Cho S., Hsu C. J., Wieneke C., Rademaker A., Weitner B. B., … Weintraub S. (2012). Dissociations between fluency and agrammatism in primary progressive aphasia. Aphasiology, 26(1), 20–43. https://doi.org/10.1080/02687038.2011.584691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. K., & Mack J. E. (2014). Grammatical impairments in PPA. Aphasiology, 28(8–9), 1018–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. K., Meltzer-Asscher A., Cho S., Lee J., Wieneke C., Weintraub S., & Mesulam M. M. (2013). Syntactic and morphosyntactic processing in stroke-induced and primary progressive aphasia. Behavioural Neurology, 26(1–2), 35–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. K., Shapiro L. P., Li L., & Schendel L. (1995). Analysis of verbs and verb-argument structure: A method for quantification of aphasic language production. Clinical Aphasiology, 23, 121–140. [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., … Joliot M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–289. https://doi.org/10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Weintraub S., Mesulam M. M., Wieneke C., Rademaker A., Rogalski E. J., & Thompson C. K. (2009). The Northwestern Anagram Test: Measuring sentence production in primary progressive aphasia. American Journal of Alzheimer's Disease and Other Dementias, 24(5), 408–416. https://doi.org/10.1177/1533317509343104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell J. L., Duffy J. R., Machulda M. M., Clark H. M., Strand E. A., Senjem M. L., … Josephs K. A. (2017). Tracking the development of agrammatic aphasia: A tensor-based morphometry study. Cortex, 90, 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell J. L., Duffy J. R., Strand E. A., Xia R., Mandrekar J., Machulda M. M., … Josephs K. A. (2013). Distinct regional anatomic and functional correlates of neurodegenerative apraxia of speech and aphasia: An MRI and FDG-PET study. Brain and Language, 125(3), 245–252. https://doi.org/10.1016/j.bandl.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. M., Dronkers N. F., Ogar J. M., Jang J., Growdon M. E., Agosta F., … Gorno-Tempini M. L. (2010). Neural correlates of syntactic processing in the nonfluent variant of primary progressive aphasia. Journal of Neuroscience, 30(50), 16845–16854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. M., Henry M. L., Besbris M., Ogar J. M., Dronkers N. F., Jarrold W., … Gorno-Tempini M. L. (2010). Connected speech production in three variants of primary progressive aphasia. Brain, 133(7), 2069–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorkston K. M., Strand E. A., Miller R., Hillel A., & Smith K. (1993). Speech deterioration in amyotrophic lateral sclerosis: Implications for the timing of intervention. Journal of Medical Speech-Language Pathology, 1(1), 35–46. [Google Scholar]