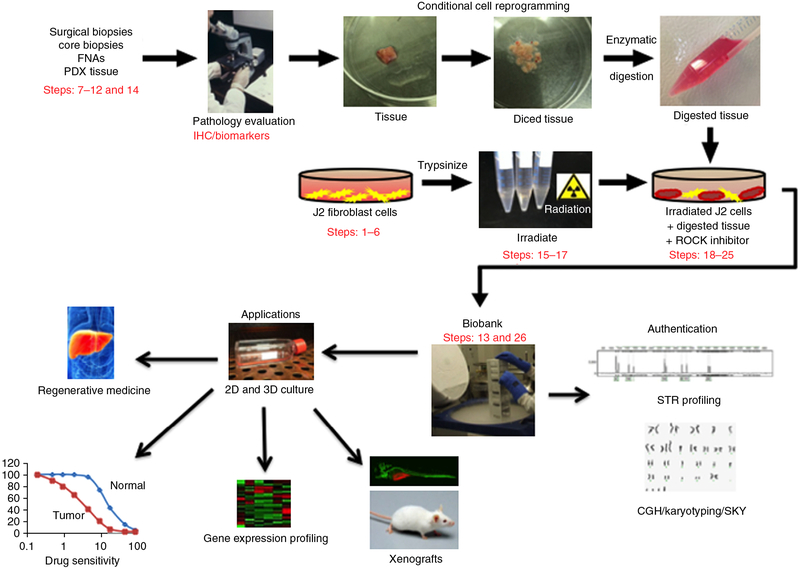
Figure 1 |.
Overview of the CR method for collection of specimens, establishment of cultures and potential applications of CR technology. Briefly, human tissue samples are obtained from surgical and core biopsies, fine-needle aspiration (FNA) or patient-derived xenograft (PDX) tissue. The samples are thoroughly evaluated by a pathologist using immunohistochemistry (IHC), and specific biomarkers are identified to ensure their normal/tumor status. CR cell lines are generated from the tissue samples using coculture with irradiated J2 feeder cells and ROCK inhibitor, and then are banked in liquid nitrogen. Subsequently, the authenticity of the cell lines should be validated by short tandem repeat (STR) profiling, comparative genomic hybridization (CGH) and spectral karyotyping (SKY). The CR cells can then be used for various applications, including regenerative medicine, drug sensitivity testing, gene expression profiling and xenograft studies. ‘Pathology evaluation’ image courtesy of V.R. Dowell (CDC; Public Health Image Library ID no. 14677). ‘Regenerative medicine’ image reproduced with permission from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. Human tissue samples were collected with the informed consent of patients, according to Georgetown University Institutional Review Board protocols.