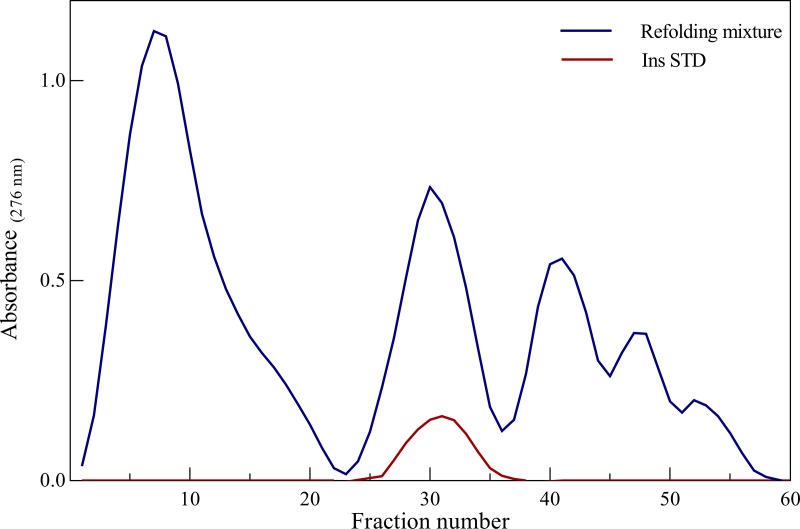
Fig 8. Purification of the insulin peptide chains on phenyl sepharose column.
The natively folded human insulin was purified using hydrophobic interaction chromatography (HIC) on a phenyl sepharose column. The elution was achieved with a reverse linear gradient of ammonium sulfate (500–0 mM) in 20 mM Tris HCl, pH 8.0. The standard insulin in the similar condition was also subjected to the same column for comparing the elution profile of the natively folded human recombinant insulin.