Fig. 6. Depleting ESCRT machinery impairs endolysosomal membrane repair.
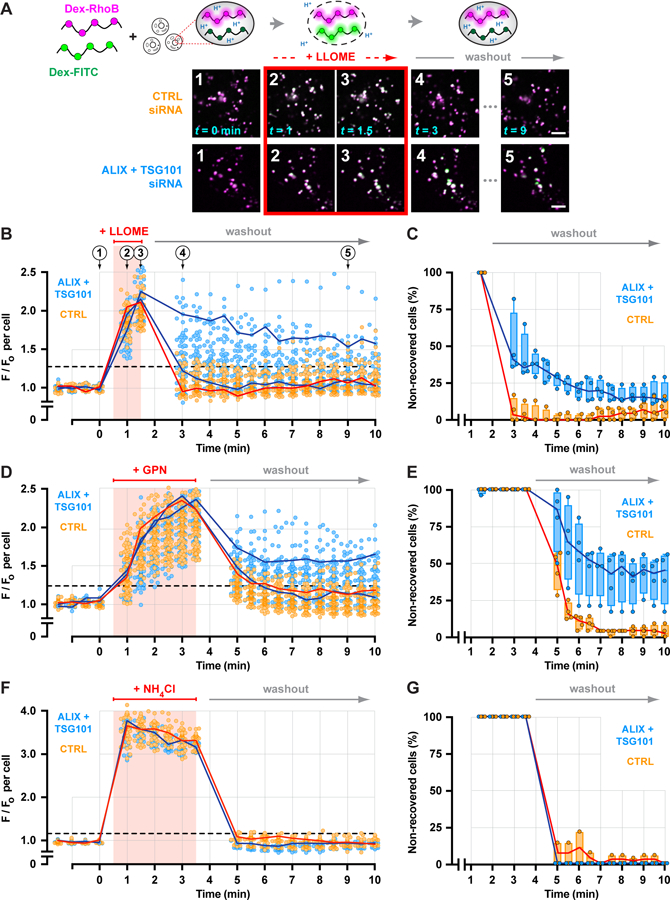
(A) A ratiometric pH-sensing assay was used to monitor endolysosomal integrity. U2OS cells transfected with the indicated siRNAs were loaded with 40–70 kDa dextrans conjugated to pH-sensitive FITC and pH-insensitive Rhodamine B fluorophores chased into endolysosomes. Loaded cells were imaged at 30-sec intervals during and after a 1-min exposure to LLOME. Fluorescence micrographs show a representative field of endolysosomes from each cohort, depicting the changes in FITC fluorescence associated with key steps in the assay. Scale bars equal 10 µm. (B) Changes in the median ratio of FITC-to-Rhodamine B fluorescence per cell at each time are plotted (n = 97 control and 85 knockdown cells from 4 experiments). Red line traces the response of a representative control cell. Blue lines designate two cells from the ESCRT-depleted cohort, one in which recovery was impaired and another in which recovery was similar to control. Horizontal dashed line specifies a recovery threshold derived from the control population (see Methods). In (C), the percentage of cells in each cohort that reached this threshold during recovery was counted, in each of 4 experiments. Lines connect the median; boxes and whiskers span the corresponding quartiles. (D-G) Using the same approach, the behavior of control and ESCRT-deficient cells was compared after a 3-min exposure to GPN (D and E; n = 99 control and 91 knockdown cells from 4 experiments) or to 10 mM ammonium chloride (F and G; n = 43 control and 29 knockdown cells from 4 experiments).
