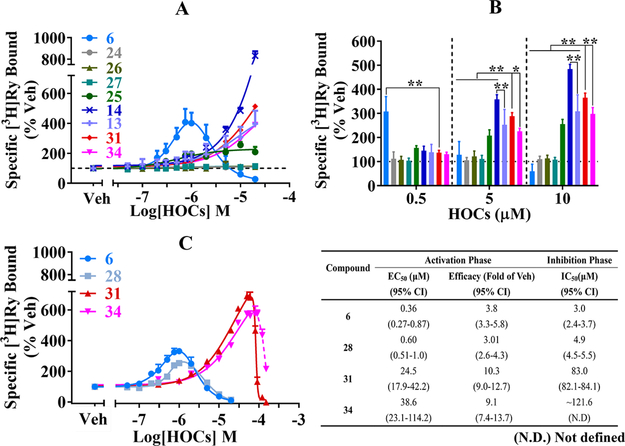
Figure 3.
Halogenated pyrroles, bipyrroles, maleimides and indoles exhibit stringent structure−activity relationship toward RyR1. Panel A shows concentration−response curves expressed as specifically bound [3H]Ry to RyR1-enriched microsomal membranes (JSR) isolated as described in Materials and Methods. Data were fitted with three-parameter or Bell-shaped (compound 6) nonlinear regression with Graph Pad 7.03. Panel B shows stimulation relative to vehicle (1% DMSO) control (% of Veh; dashed line). Panel C shows results from an expanded concentration range to quantify biphasic parameters fitted as described in panel A for compounds 6, 28, and 31. The activation phase for 34 was fitted with a threeparameter equation but lacked sufficient data to fit the inhibition phase (dashed line). Parameters obtained from curve-fitting are summarized in inserted table. Data shown are from 2 to 3 independent preparations conducted in triplicates or quintuplicates and expressed as Mean ± SD. With Graph Pad 7.03, one-way ANOVA followed by Tukey’s multiple comparisons test was used to determine the significance among specific compounds. * p < 0.05 and ** p < 0.01.