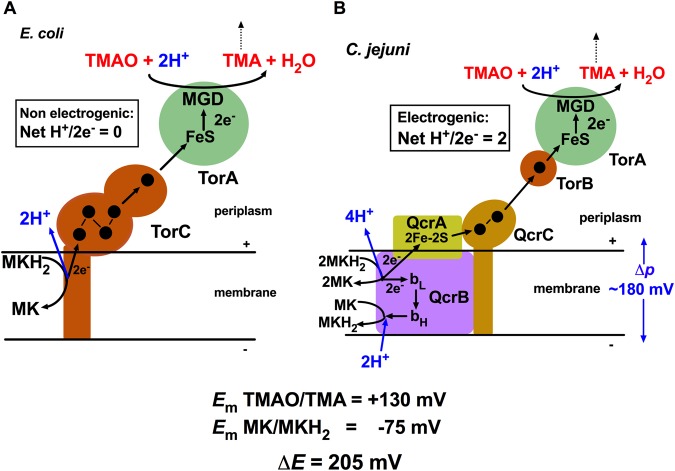
Figure 7.
Comparison of the mechanism of TMAO reduction in E. coli and C. jejuni. (A) shows how quinol oxidation on the periplasmic side of the membrane in E. coli is coupled to electron transfer (black arrows) through the five haems in TorC (black filled circles) and the [4Fe-4S] and molybdopterin guanine dinucleotide (MGD) cofactors in TorA, ultimately reducing TMAO to TMA. Note that this mechanism is not energy-conserving (H+/2e− transferred is 0) because proton release from quinol oxidation and proton uptake during TMAO reduction occur in the same compartment (the periplasm). (B) shows the proposed energy-conserving mechanism of TMAO reduction in C. jejuni coupled to the proton-motive Q cycle in the Qcr complex, which gives an overall net H+/2e− ratio of 2. Although the redox span between menaquinol and TMAO is 205 mV, close to the typical value of ~180 mV for Δp, continued diffusion of TMA away from the system (dashed black arrow) would shift the equilibrium and favour electrogenic TMAO reduction. TorB (Cj0265), homologous to the C-terminal domain of TorC (see Supplementary Fig. 2), most likely receives electrons directly from QcrC (black arrows), before transfer to the redox centres in TorA. bL, low-potential haem b; bH, high-potential haem b in the QcrB subunit.