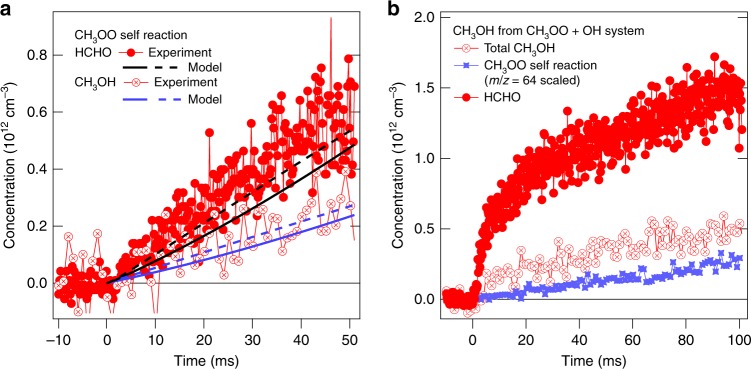
Fig. 1.
Formaldehyde and methanol time profiles from the methylperoxy self- and hydroxyl reactions. Comparison of the contributions from 13CH3OO self-reaction and reaction of 13CH3OO with OH in producing methanol in the photolysis experiments at P = 30 Torr. a CH3OO self-reaction (photolysis of 13CH3I in the presence of O2) compared to a kinetic model employing literature rate coefficients and directly measured reactant concentrations, wall loss and two fits to the photolytic depletion. b Measurements at the same conditions as (a) except with the addition of H2O2. The contribution from 13CH3OO self-reaction is represented by the signal from another product at m/z = 64, 13CH3OO13CH3 (formed only by the self-reaction), scaled using directly measured branching fractions of the self-reaction. The additional, rapidly formed 13CH3OH arises from the reactions of 13CH3OO with OH and 13CH3O with HO2. The temporal resolution of the methanol and CH3OOCH3 signals is here reduced by a factor of five to more clearly show the amplitudes