Abstract
Objective
To determine rates of gastrostomy (GT) in very low birth weight (VLBW) infants.
Study Design
Retrospective, cross-sectional analysis of the Kids’ Inpatient Database for the years 2000, 2003, 2006, 2009 and 2012. We identified VLBW births and infants undergoing a gastrostomy (GT), with and without fundoplication, using ICD-9-CM codes.
Result
National rates (per 1000 VLBW births) of GT increased from 11.5 GT (95% CI, 10-13) in 2000 to 22.9 (95% CI, 20-25) in 2012 (p<0.001). Gastrostomy with and without fundoplication increased during the study period (p<0.001 in both groups). VLBW survival also increased from 78.5% in 2000 to 81.1% in 2012 (p<0.001). In all study years, the Northeast census region had the lowest GT rates; while, the West had the highest rates in four of the five study years.
Conclusion
Between 2000 and 2012, the incidence of GT in VLBW infants doubled, associated with improvements in survival in this population.
Introduction
Advances in antenatal maternal and neonatal care in the last several decades have led to marked improvements in survival of premature infants.1–4 Very low birth weight (VLBW) infant in-hospital mortality in US centers participating in the Vermont Oxford Network (representing 756 centers in 2014) nearly halved from 18.1% in 19911 to 10.9% in 2014.3 Among VLBW infants, the largest relative increase in survival has come in infants at the limits of viability (23-25 weeks).2, 4 Despite national improvements in morbidities in VLBW infants,3 improved rates of survival have led to more infants surviving with major morbidities of prematurity, particularly in infants at the limits of viability.2
Very low birth weight infants, especially those born at the limits of viability or with significant respiratory disease, are at risk for feeding/swallowing dysfunction and postnatal growth failure.5–8 Approximately 50% of extremely low birth weight infants require assistance with feeding at 18 months of age.7 Surgical placement of a gastrostomy (GT) tube is often used in infants with neurologic impairment, feeding dysfunction and growth failure to ensure adequate long-term nutrition.9, 10 Although GT is the most common gastrointestinal operation performed in neonates,11 the impact of increasing survival of VLBW infants on the national utilization of GT has not been reported. Furthermore, the safety and efficacy of GT placement, including which infants would benefit from this therapy, is unknown.
We hypothesized that coincident with improving national VLBW survival, GT placement also increased. The main objective of our study was to describe the national trends in the utilization of GT as a long-term feeding modality in VLBW infants in a nationally representative sample of all VLBW births. Secondarily, we evaluated the regional variation in the use of GT in this population.
Methods
Study Design and Data Sources
We performed a retrospective, cross-sectional analysis of pediatric discharge data from 2000, 2003, 2006, 2009, and 2012 (the most recent year available), from the Healthcare Cost and Utilization Project’s (HCUP) Kids’ Inpatient Database (KID), compiled by the Agency for Healthcare Research and Quality (AHRQ). The KID is a nationally representative dataset that samples 80% of pediatric inpatient discharges and 10% of uncomplicated in-hospital births to allow evaluations of rare diseases or treatments in children.12–14 Each patient record contains a discharge weight to allow calculation of national and regional rates of diagnoses and procedures using a specified sampling method. Because our study utilized de-identified data it was deemed exempt by the Vanderbilt University Medical Center Institutional Review Board.
Identification of Study Population
We identified VLBW infants using a combination of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes15 (Appendix 1) and All-Payer Severity-Adjusted Diagnosis Related Groups (DRG), version 18 or 24, depending on the study year.16 Infants who had an ICD-9-CM code for a birth weight less than 1500 grams and did not have a DRG code (389-391) associated with term birth or normal neonates were included in the study cohort. We did not exclude infants based upon postnatal age at the time of hospital admission. We used the hospital birth variable to identify the number of VLBW infants born per study year since some VLBW infants would have been born at one facility, transferred to another hospital for Neonatal Intensive Care Unit (NICU) care and thus potentially be represented twice in the KID.
Demographics and Comorbidities
We utilized patient, payer and hospital characteristics provided by the KID to describe the study population. Primary payers were grouped into three categories: public sources (Medicaid and Medicare), private insurance, and other (includes self-pay, no charge and other sources). The KID contains hospital information including region, teaching status and urban vs. rural location that were included in the data collection. Multiple infant comorbidities including bronchopulmonary dysplasia (BPD), severe intraventricular hemorrhage (IVH)- Grade 3 or 4, and severe retinopathy of prematurity (ROP)- grades 3-5 were evaluated. Further, we assessed infants for one of the neuromuscular complex chronic conditions (CCC) as described by Feudtner et al.17
Study Outcomes
The primary outcome of the study was the receipt of a GT. Gastrostomy placement was identified using ICD-9-CM procedure codes 43.11 (Percutaneous [endoscopic] gastrostomy) and 43.19 (other gastrostomy).18 We further classified infants according to whether they had received a fundoplication (ICD-9-CM procedure codes 44.66 [other procedures for creation of esophagogastric sphincteric competence] and 44.67 [laparoscopic procedures for creation of esophagogastric sphincteric competence]) during the same hospitalization as their GT.11 As Current Procedural Terminology (CPT) codes are not available in the KID, we used ICD-9-CM procedure codes alone to identify the procedures and thus could not reliably identify open versus laparoscopic GT.
Statistical Analysis
We utilized survey weights in the KID to calculate national rates of GT, GT only and GT with fundoplication (GTF) for all study years. We used the number of infants with procedure codes for the respective procedures as the numerator and the number of in-hospital VLBW infant live births for each study year as the denominator. Rates were described per 1000 VLBW live births. In addition to national rates, we calculated regional and birth weight specific rates of GT. To evaluate potential explanations for trends in GT utilization over time, we calculated national and birth weight specific survival for all study years. We evaluated statistical significance of secular trends for all rates over time by assessing P for trend using variance weighted least squares regression with study year as the independent variable in the models.14
We calculated descriptive statistics for the populations of infants discharged during 2012 who received no GT, GT only or GTF. We compared differences in characteristics of infants who received GT only versus GTF using Pearson’s chi-square test. To evaluate for changes in the population of infants who received GT over the study period, we calculated descriptive statistics for infants who received GT during each of the study years and assessed P for trend using variance weighted least squares regression with study year as the independent variable in the models.14
Finally, using data from 2012, we built a multivariable logistic regression model to identify factors associated with GT in VLBW infants. The dependent variable for the model was a GT placement and the a priori chosen independent variables included sex, birth weight, neonatal comorbidities (severe IVH, surgically-ligated patent ductus arteriosus, BPD, aspiration and chromosomal anomalies), payer, hospital characteristics and census region. Variables were chosen a priori to preserve the Type 1 error of the model.19 Since the KID is de-identified, it is impossible to follow infants who were transferred across multiple hospitalizations. Therefore, to construct our regression model, we used all 2012 VLBW hospitalizations instead of all 2012 VLBW births, as some infants would not have received their GT at their birth hospital. All analyses were completed with STATA version 14.1 (StataCorp LP, College Station, TX) using discharge survey weights provided in the datasets by the AHRQ.20 P-values less than 0.05 were considered significant.
Results
During the five study years, a total of 253 117 VLBW infants were born in the US with 4060 (1.6%) infants receiving a GT. A total of 2397/4060 (59%) infants received GT only and 1663/4060 (41%) received GTF. National rates (per 1000 VLBW births) increased significantly over the study period from 11.5 GT in 2000 to 22.9 in 2012 (p<0.001) (Figure 1). Rates for all study years are shown in Table 1. In all study years, the lowest GT rates occurred in the Northeast Census Region while the highest rates in four of the five study years occurred in the West (Figure 2).
Figure 1.
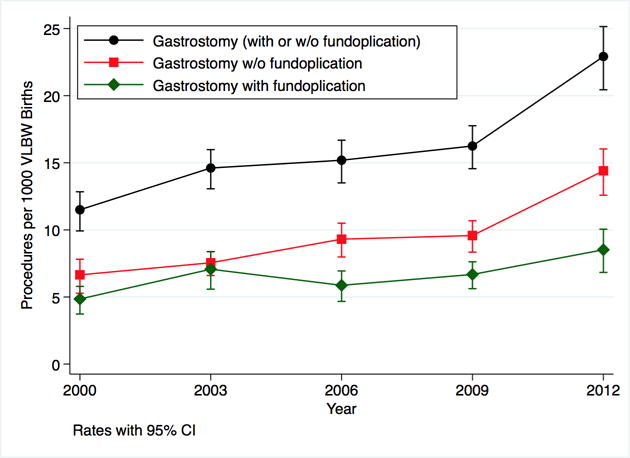
Nationally representative rates of all gastrostomy (black), gastrostomy only (red) and gastrostomy with fundoplication (green) per 1000 very low birth weight (VLBW) infants using weighted estimates of procedures and VLBW live births in the Kids’ Inpatient Database 2000, 2003, 2006, 2009 and 2012.
Table 1.
Estimated National and Regional Rates of Gastrostomy and Gastrostomy/Fundoplication1
| 2000 | 2003 | 2006 | 2009 | 2012 | P for trend | |
|---|---|---|---|---|---|---|
| VLBW births | 49561 (45253–53868) |
48736 (45777–51695) |
54279 (51055–57503) |
52623 (49686–55561) |
47918 (45358–50479) |
– |
| Gastrostomy procedures | 570 (449–692) |
712 (598–826) |
824 (689–959) |
855 (724–987) |
1098 (927–1270) |
– |
| Rate of procedure(s) per 1000 VLBW live births (95% confidence intervals) | ||||||
| Gastrostomy (all) | 11.5 (10–13) |
14.6 (13–16) |
15.2 (14–17) |
16.3 (15–18) |
22.9 (20–25) |
<0.001 |
| Gastrostomy only | 6.7 (5–8) |
7.6 (7–8) |
9.3 (8–10) |
9.6 (8–11) |
14.4 (13–16) |
<0.001 |
| Gastrostomy with fundoplication | 4.8 (4–6) |
7.1 (6–8) |
5.9 (5–7) |
6.7 (6–8) |
8.5 (7–10) |
<0.001 |
| Rates of gastrostomy (95% CI) by census region | ||||||
| Northeast | 7.9 (6–10) |
7.7 (5.6–9.3) |
9.3 (7–11) |
11.8 (9–14) |
16 (11–20) |
<0.001 |
| Midwest | 12.5 (8–15) |
15.4 (13–17.6) |
16.6 (12–20) |
19.2 (15–22) |
24.1 (19–28) |
<0.001 |
| South | 10.6 (8–13) |
15.7 (13–18) |
15 (12–17) |
14.1 (11–17) |
25.5 (21–29) |
<0.001 |
| West | 15.4 (11–19) |
18.1 (14–22) |
19.3 (15–23) |
21.5 (17–25) |
22.3 (17––7) |
<0.001 |
For all the rates in the table, the numerator is the number procedures (either nationally or in the respective regions) and the denominator is the number of very low birth weight (VLBW) infants (either nationally or in the respective regions).
Figure 2.
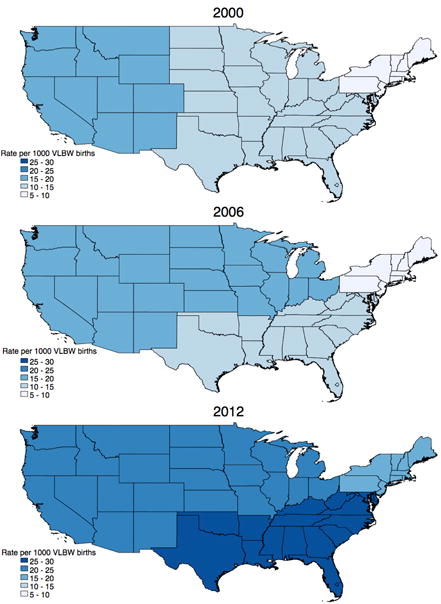
Changes in the rate of gastrostomy per 1000 very low birth weight (VLBW) births by US Census Regions, using weighted estimates in the Kids’ Inpatient Database 2000, 2006 and 2012
When stratified by birth weight, infants less than 500 grams showed the largest relative increase in GT (4.9 GT/1000 births in 2000 to 14.1 GT/1000 births in 2012), however the rates of GT in all three birth weight strata increased significantly (p<0.001). Infants with birth weight 500-999 grams were the primary factor in the national increase in the rate of GT placement (17.9 GT/1000 births in 2000 to 39.2 GT/1000 births in 2012). In parallel with the GT rates, survival for all three birth weight strata increased significantly over the study years (p<0.001) (Table 2).
Table 2.
Gastrostomy Status and Survival by Birth Weight
| 2000 | 2003 | 2006 | 2009 | 2012 | P for trend | |
|---|---|---|---|---|---|---|
| All VLBW Infants | ||||||
| In-hospital live births | 49561 | 48736 | 54279 | 52623 | 47918 | – |
| In-hospital deaths | 10643 | 10924 | 11513 | 10554 | 9052 | – |
| Survival to hospital discharge (%) | 78.5 | 77.6 | 78.8 | 79.9 | 81.1 | <0.001 |
| Gastrostomies | 570 | 712 | 824 | 855 | 1098 | – |
| Gastrostomies/1000 births | 11.5 | 14.6 | 15.2 | 16.3 | 22.9 | <0.001 |
| < 500 grams | ||||||
| In-hospital live births | 4511 | 4599 | 4854 | 4799 | 4455 | – |
| In-hospital deaths | 4077 | 4166 | 4433 | 4293 | 3874 | – |
| Survival to hospital discharge (%) | 9.6 | 9.4 | 8.7 | 10.5 | 13 | <0.001 |
| Gastrostomies | 22 | 19 | 39 | 41 | 63 | – |
| Gastrostomies/1000 births | 4.9 | 4.1 | 8.0 | 8.5 | 14.1 | <0.001 |
| 500–999 grams | ||||||
| In-hospital live births | 19259 | 18550 | 20323 | 19408 | 17925 | – |
| In-hospital deaths | 5437 | 5674 | 5827 | 5052 | 4198 | – |
| Survival to hospital discharge (%) | 71.8 | 69.4 | 71.3 | 74 | 76.6 | <0.001 |
| Gastrostomies | 344 | 455 | 519 | 546 | 702 | – |
| Gastrostomies/1000 births | 17.9 | 24.5 | 25.5 | 28.1 | 39.2 | <0.001 |
| 1000–1499 grams | ||||||
| In-hospital live births | 25791 | 25587 | 29102 | 28416 | 25539 | – |
| In-hospital deaths | 1135 | 1084 | 1253 | 1209 | 981 | – |
| Survival to hospital discharge (%) | 95.6 | 95.8 | 95.7 | 95.7 | 96.2 | 0.004 |
| Gastrostomies | 203 | 238 | 266 | 268 | 334 | – |
| Gastrostomies/1000 births | 7.9 | 9.3 | 9.1 | 9.4 | 13.1 | <0.001 |
The rates of VLBW infants receiving GT only and GTF increased over the study years (p<0.001). The population of infants who received GT only and those who received GTF were relatively similar (Supplemental Table 1, Online Appendix). In univariate analysis, infants who received GTF had lower gestational age at birth (p<0.04) and were more likely to have a diagnosis of gastroesophageal reflux at time of discharge (p<0.001). Death prior to discharge was higher in the GT only infants (6% versus <1%, p=0.02).
Characteristics of infants receiving a GT changed over the study period (Supplemental Table 2, Online Appendix). Compared to infants who received a GT in 2000, infants who received a GT in 2012 were more likely to receive public insurance (p<0.001) and to receive their GT in an urban teaching hospital versus an urban non-teaching hospital (p<0.001). The rate of tracheostomy in infants receiving a GT declined over the study period (23% in 2000 to 13% in 2012, p<0.001), as did the in-hospital mortality of infants receiving a GT (11% in 2000 to 4% in 2012, p<0.001). The percentages of infants with a neuromuscular CCC and chromosomal anomalies increased significantly over the study years. The incidences of BPD, severe IVH and PVL in infants receiving a GT did not significantly change.
After accounting for potential confounders, infants with significant comorbidities including surgically ligated PDA (adjusted odds ratio [aOR] 2.1, 95% confidence intervals [CI] 1.7-2.6) and BPD (aOR 4.1, 95% CI 3.3-5.1) were more likely to have received a GT. Furthermore, significant differences in regional utilization of GT existed even after adjustment for potential confounders with infants hospitalized in the South (aOR 1.6, 95% CI 1.1- 2.4) and West (aOR 1.6, 95% 1.0-2.5) regions more likely to receive a GT (Supplemental Table 3, Online Appendix).
Discussion
Our study demonstrates increasing use of GT, both with and without fundoplication, for long-term feeding of VLBW infants in the US. From 2000-2012, the national rate of GT placement in VLBW infants doubled. Although the increase was most prominent in infants <500 grams, we found significant increases among all VLBW infants. We speculate that the improving rate of survival for VLBW infants resulted in a greater proportion of infants with dysphagia due to immaturity or neurologic injury and may partially explain the increase in GT placement. We also demonstrated significant regional practice variation, even after adjustment for multiple infant characteristics and comorbidities, with the lowest rates of GT consistently found in the Northeast region.
Though our study is the first to characterize the epidemiology of GT in VLBW infants, our findings are consistent with previous work that showed an increase in the national use of GT in children. Using the KID, Fox et al. showed that the rate of GT placement in pediatric patients (<18 years of age) increased from 16.6 GT/100,000 children in 1997 to 18.5 GT/100,000 children in 2009. Children <1 year of age primarily accounted for the overall increase in GT (136.6 GT/100,000 children <1 year in 1997 to 173 GT/100,000 children <1 year in 2009). Also consistent with our results, these authors found that children born prematurely made up a larger percentage of the total GT population over time (11.5% in 1997 to 15% in 2009, p<0.001).21 Berry et al. used the KID to show similar findings of increased rates of GT in pediatric patients (<18 years of age) from 1997-2006 for children with and without a diagnosis associated with neurologic impairment.22
From our data, we are unable to determine with certainty the cause of the increasing rate of GT in VLBW infants. We suggest, based on the available data (Table 2), that improved survival of VLBW infants over the study period may be a key driver of this increase. In support of this proposal, we found the largest increases in GT rates were seen in the birth strata with the highest relative improvements in survival (<500 grams and 500-999 grams). During the study period, no consensus guidelines or studies were published to change clinical practice for GT placement in VLBW infants. In 2006, the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition published a national guideline that advocated consideration for earlier placement of GT in neurologically impaired children.23 However, our data show increases in GT prior to the publication of this guideline. Other potential explanations for the increase in GT include more availability of pediatric surgical specialists (supply-sensitive care), advances in the use of laparoscopic GT surgery making GT a potentially less invasive procedure, earlier placement of GT due to payer pressures/shorter hospitalizations and/or changes in provider bias/preference in recommending GT.
Interventions for long-term nutrition in former preterm infants with feeding dysfunction include home nasogastric (NG) tubes, GT placement or GTF. The long-term safety and efficacy of each of these interventions in preterm infants are not well known. Home NG feeding, in association with home nursing support, has been shown to be safe and allow earlier discharge of preterm infants;24 however, NG feeds as a long-term home feeding modality in preterm infants is not well-studied. Gastrostomy feeding has been shown to improve growth9, 25, 26 and decrease caregiver stress27 in older children, but has also been associated with increased emergency department visits and re-hospitalizations.28 Given limitations of the dataset, we were unable to assess how the epidemiology of home NG tubes changed over the course of the study years as the use of GT increased. This is an important area for future prospective study.
Developing evidence and guidelines to guide decision-making for GT is especially important for VLBW infants, as surgical procedures themselves have been independently associated with poorer long-term outcomes in preterm infants.29 Currently, data to inform which neonates would benefit from GT placement or GTF are limited. Likely as a result of this limited evidence, we found significant regional practice variation even after accounting for patient-level factors (Table 1). Given the data structure of the KID, we were not able to reliably define variation at the hospital level. It is possible that the regional variation in GT rates is explained by home NG tube use or increased rates of GT after discharge in the Northeast region. Given the importance of postnatal growth on neurodevelopmental outcomes30 and cardiovascular and metabolic risk profiles later in life,31 well-designed prospective studies assessing growth, neurodevelopmental outcomes, feeding trajectories and complications (both surgical and medical) of these therapies are essential.
Although our study provides the first understanding of the national epidemiology of GT in VLBW infants, it also has some important limitations. Due to the de-identified data structure of the KID,20 we were unable to follow infants that were transferred from one center to another. This makes it difficult to determine the timing of GT during the hospitalization in many infants as well as the age of the infant at the time of the procedure. As these administrative data were collected at discharge, we are also unable to determine temporality with other acquired morbidities such as IVH during the hospitalization. This complicates the interpretation of our regression analysis because some of the comorbidities that were used as covariates may not have been present at the time of GT, or even resulted from the actual procedure itself. We also observed a difference in mortality in the GT and GTF groups that likely represents selection bias and not a consequence of the operative procedures themselves. Infants with higher pre-operative morbidity may have preferentially received GT versus GTF due to the more invasive nature of GTF and the additional operative risks. As with all studies utilizing administrative data we are unable to determine causality for the temporal increase in GT, only hypothesize about possible causes for the associations seen. Lastly, the potential exists for misclassification of outcomes due to coding errors.
In summary, the national rate of GT placement in VLBW infants doubled from 2000-2012, associated with improving rates of survival in this population. Given the paucity of data regarding safety, efficacy and long-term outcomes of preterm infants treated with GT, prospective studies to determine the optimal long-term feeding strategies for preterm infants after NICU discharge are essential. As preterm survival continues to improve and the absolute number of preterm infants with significant morbidity including feeding dysfunction increases these investigations will become increasingly important.
Supplementary Material
Acknowledgments
We would like to acknowledge and thank Ann R. Stark, MD for her editorial assistance with the writing of this manuscript.
Funding Source: Research reported in this publication was supported by the Katherine Dodd Faculty Scholars program (Hatch) and the National Institute On Drug Abuse of the National Institutes of Health under award number K23DA038720 (Patrick). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Abbreviations
- AHRQ
Agency for Healthcare Research and Quality
- DRG
Diagnosis Related Groups
- BPD
bronchopulmonary dysplasia
- CCC
complex chronic conditions
- GT
gastrostomy
- HCUP
Healthcare Cost and Utilization Project
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IVH
intraventricular hemorrhage
- KID
Kids’ Inpatient Database
- NG
nasogastric
- NICU
Neonatal Intensive Care Units
- ROP
retinopathy of prematurity
- VLBW
very low birth weight
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have no conflicts of interest relevant to this article to disclose.
References
- 1.Horbar JD, Badger GJ, Carpenter JH, Fanaroff AA, Kilpatrick S, LaCorte M, et al. Trends in mortality and morbidity for very low birth weight infants, 1991-1999. Pediatrics. 2002;110(1 Pt 1):143–151. doi: 10.1542/peds.110.1.143. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. JAMA. 2015;314(10):1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horbar JD, Edwards EM, Greenberg LT, Morrow KA, Soll RF, Buus-Frank ME, et al. Variation in Performance of Neonatal Intensive Care Units in the United States. JAMA Pediatr. 2017;171(3):e164396. doi: 10.1001/jamapediatrics.2016.4396. [DOI] [PubMed] [Google Scholar]
- 4.Younge N, Goldstein RF, Bann CM, Hintz SR, Patel RM, Smith PB, et al. Survival and Neurodevelopmental Outcomes among Periviable Infants. N Engl J Med. 2017;376(7):617–628. doi: 10.1056/NEJMoa1605566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizuno K, Nishida Y, Taki M, Hibino S, Murase M, Sakurai M, et al. Infants with bronchopulmonary dysplasia suckle with weak pressures to maintain breathing during feeding. Pediatrics. 2007;120(4):e1035–1042. doi: 10.1542/peds.2006-3567. [DOI] [PubMed] [Google Scholar]
- 6.Lee JH, Chang YS, Yoo HS, Ahn SY, Seo HJ, Choi SH, et al. Swallowing dysfunction in very low birth weight infants with oral feeding desaturation. World J Pediatr. 2011;7(4):337–343. doi: 10.1007/s12519-011-0281-9. [DOI] [PubMed] [Google Scholar]
- 7.Adams-Chapman I, Bann C, Carter SL, Stoll BJ. Language outcomes among ELBW infants in early childhood. Early Hum Dev. 2015;91(6):373–379. doi: 10.1016/j.earlhumdev.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malkar MB, Gardner W, Welty SE, Jadcherla SR. Antecedent Predictors of Feeding Outcomes in Premature Infants With Protracted Mechanical Ventilation. J Pediatr Gastroenterol Nutr. 2015;61(5):591–595. doi: 10.1097/MPG.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 9.Corwin DS, Isaacs JS, Georgeson KE, Bartolucci AA, Cloud HH, Craig CB. Weight and length increases in children after gastrostomy placement. J Am Diet Assoc. 1996;96(9):874–879. doi: 10.1016/s0002-8223(96)00239-8. [DOI] [PubMed] [Google Scholar]
- 10.Guimber D, Michaud L, Storme L, Deschildre A, Turck D, Gottrand F. Gastrostomy in infants with neonatal pulmonary disease. J Pediatr Gastroenterol Nutr. 2003;36(4):459–463. doi: 10.1097/00005176-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Barnhart DC, Hall M, Mahant S, Goldin AB, Berry JG, Faix RG, et al. Effectiveness of fundoplication at the time of gastrostomy in infants with neurological impairment. JAMA Pediatr. 2013;167(10):911–918. doi: 10.1001/jamapediatrics.2013.334. [DOI] [PubMed] [Google Scholar]
- 12.Healthcare Cost and Utilization Project (HCUP) 2000, 2006, 2009, 2012 Overview of the Kids’ Inpatient Database (KID) https://www.hcup-us.ahrq.gov/kidoverview.jsp. Accessed May 4, 2017.
- 13.Patrick SW, Davis MM, Lehmann CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol. 2015;35(8):650–655. doi: 10.1038/jp.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA. 2012;307(18):1934–1940. doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC) International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) https://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed January 21, 2017.
- 16.Ingenix. All-Payer Severity-Adjusted Diagnosis Related Groups (APS-DRGS ®) Assignment. https://www.hcup-us.ahrq.gov/db/nation/nis/APS-Def_Public_v24.pdf. Published 2008. Accessed January 21, 2017.
- 17.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6):E99. doi: 10.1542/peds.107.6.e99. [DOI] [PubMed] [Google Scholar]
- 18.Miyata S, Dong F, Lebedevskiy O, Park H, Nguyen N. Comparison of operative outcomes between surgical gastrostomy and percutaneous endoscopic gastrostomy in infants. J Pediatr Surg. 2017 doi: 10.1016/j.jpedsurg.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Harrell FE. Regression Modeling Strategies with Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Springer-Verlag; Cham: 2015. pp. 67–72. [Google Scholar]
- 20.2012 Healthcare Cost and Utilization Project (HCUP) Introduction to the HCUP KIDS' Inpatient Database (KID) 2012 https://www.hcup-us.ahrq.gov/db/nation/kid/KID_2012_Introduction.pdf. Published 2014.
- 21.Fox D, Campagna EJ, Friedlander J, Partrick DA, Rees DI, Kempe A. National trends and outcomes of pediatric gastrostomy tube placement. J Pediatr Gastroenterol Nutr. 2014;59(5):582–588. doi: 10.1097/MPG.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 22.Berry JG, Poduri A, Bonkowsky JL, Zhou J, Graham DA, Welch C, et al. Trends in resource utilization by children with neurological impairment in the United States inpatient health care system: a repeat cross-sectional study. PLoS Med. 2012;9(1):e1001158. doi: 10.1371/journal.pmed.1001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchand V, Motil KJ. Nutrition support for neurologically impaired children: a clinical report of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2006;43(1):123–135. doi: 10.1097/01.mpg.0000228124.93841.ea. [DOI] [PubMed] [Google Scholar]
- 24.Ortenstrand A, Waldenstrom U, Winbladh B. Early discharge of preterm infants needing limited special care, followed by domiciliary nursing care. Acta Paediatr. 1999;88(9):1024–1030. doi: 10.1080/08035259950168568. [DOI] [PubMed] [Google Scholar]
- 25.Avitsland TL, Birketvedt K, Bjornland K, Emblem R. Parent-reported effects of gastrostomy tube placement. Nutr Clin Pract. 2013;28(4):493–498. doi: 10.1177/0884533613486484. [DOI] [PubMed] [Google Scholar]
- 26.Cook S, Hooper V, Nasser R, Larsen D. Effect of gastrostomy on growth in children with neurodevelopmental disabilities. Can J Diet Pract Res. 2005;66(1):19–24. doi: 10.3148/66.1.2005.19. [DOI] [PubMed] [Google Scholar]
- 27.Avitsland TL, Faugli A, Pripp AH, Malt UF, Bjornland K, Emblem R. Maternal psychological distress and parenting stress after gastrostomy placement in children. J Pediatr Gastroenterol Nutr. 2012;55(5):562–566. doi: 10.1097/MPG.0b013e31826078bd. [DOI] [PubMed] [Google Scholar]
- 28.Goldin AB, Heiss KF, Hall M, Rothstein DH, Minneci PC, Blakely ML, et al. Emergency Department Visits and Readmissions among Children after Gastrostomy Tube Placement. J Pediatr. 2016;174:139–145 e132. doi: 10.1016/j.jpeds.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 29.Morriss FH, Jr, Saha S, Bell EF, Colaizy TT, Stoll BJ, Hintz SR, et al. Surgery and neurodevelopmental outcome of very low-birth-weight infants. JAMA Pediatr. 2014;168(8):746–754. doi: 10.1001/jamapediatrics.2014.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franz AR, Pohlandt F, Bode H, Mihatsch WA, Sander S, Kron M, et al. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics. 2009;123(1):e101–109. doi: 10.1542/peds.2008-1352. [DOI] [PubMed] [Google Scholar]
- 31.Hack M, Schluchter M, Cartar L, Rahman M, Cuttler L, Borawski E. Growth of very low birth weight infants to age 20 years. Pediatrics. 2003;112(1 Pt 1):e30–38. doi: 10.1542/peds.112.1.e30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


