Figure 5.
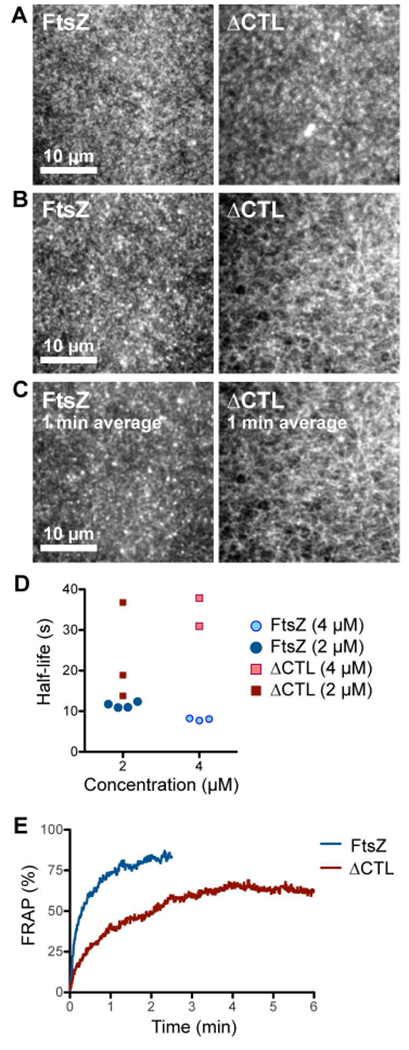
ΔCTL forms stable networks of straight filaments unlike WT FtsZ. A, B. Contrast enhanced micrographs of structures formed on SLBs at steady state after simultaneously flowing in 4 μM FtsZ (6% Alexa488 labeled) and 4 μM FtsZ-MTS (unlabeled) or 4 μM ΔCTL (6% Alexa488 labeled) and 4 μM ΔCTL-MTS (unlabeled) in the protein channel inlet and 4 mM GTP in the GTP channel inlet and stopping flow. A. Structures formed farther from the original laminar boundary on the protein side. B. Structures formed closest to the original laminar boundary on the protein side. C. Time averages corresponding to the structures shown in B. obtained from taking averages over frames corresponding to a 1-minute time interval. Scale bar – 10 μm. D. Time until decrease in fluorescence intensity to half-maximum value (half-life) for structures formed by FtsZ (6% Alexa488 labeled) and FtsZ-MTS (unlabeled) or ΔCTL (6% Alexa488 labeled) and ΔCTL-MTS (unlabeled), following depletion of GTP. Half-life values were estimated from non-linear fits assuming one-phase exponential decay. E. Fluorescence recovery after photobleaching corresponding to structures showed in A (on the protein side, away from the original laminar boundary). Plot shows average of 3 replicates. Reaction buffer contains 50 mM HEPES pH 8.0, 0.1 mM EDTA, 10 mM MgCl2, 300 mM KCl, 1% glycerol, 0.1 mg mL−1 casein (blocking agent), 0.5 mg mL−1 ascorbate.
