Abstract
Objectives:
Ultrasonography has shown promising diagnostic value in dental implant imaging research; however, exactly how ultrasound was used and at what stage of implant therapy it can be applied has not been systematically evaluated. Therefore, the aim of this review is to investigate potential indications of ultrasound use in the three implant treatment phases, namely planning, intraoperative and post-operative phase.
Methods:
Eligible manuscripts were searched in major databases with a combination of keywords related to the use of ultrasound imaging in implant therapy. An initial search yielded 414 articles, after further review, 28 articles were finally included for this systematic review.
Results:
Ultrasound was found valuable, though at various development stages, for evaluating (1) soft tissues, (2) hard tissues (3) vital structures and (4) implant stability. B-mode, the main function to image anatomical structures of interest, has been evaluated in pre-clinical and clinical studies. Quantitative ultrasound parameters, e.g. sound speed and amplitude, are being developed to evaluate implant-bone stability, mainly in simulation and pre-clinical studies. Ultrasound could be potentially useful in all three treatment phases. In the planning phase, ultrasound could evaluate vital structures, tissue biotype, ridge width/density, and cortical bone thickness. During surgery, it can provide feedback by identifying vital structures and bone boundary. At follow-up visits, it could evaluate marginal bone level and implant stability.
Conclusions:
Understanding the current status of ultrasound imaging research for implant therapy would be extremely beneficial for accelerating translational research and its use in dental clinics.
Introduction
The use of dental implants to replace missing dentition is rapidly increasing and has become the standard of care owing to the high survival rate.1, 2 Successful implant treatment requires prudent clinical evaluation and high-quality images of the surgical site. An ideal imaging modality should provide sufficient anatomical information pertinent to the implant site and cause no harm, in addition to being easy to use and low cost.3 Currently, two-dimensional (2D) imaging modalities including panoramic films and intraoral radiographs are the most commonly used. However, image magnification/distortion and the lack of cross-sectional information, etc are among the major disadvantages.4 During the past decade, the use of cone beam CT (CBCT) is on a rise.5 The American Academy of Oral and Maxillofacial Radiology recommends that evaluation of a potential implant site should include cross-sectional imaging, orthogonal to the site of interest.6 Being the only reasonable form of cross-sectional imaging tool commonly available to clinicians, CBCT potentially offers a more accurate diagnosis and aids with the successful management of a wide range of clinical challenges, such as placements of multiple implants and major reconstructive surgeries.7–9 In addition to planning for implant surgery, CBCT enables a digital workflow for surgical guide and restoration fabrication.10 However, errors in acquiring the image, designing virtual implant location and fabricating the guide can compound and unbeknown reach clinical significance in critical cases during surgery. Malpositioned implants have a higher risk for developing post-operative complications including bone loss and mucosal recession. Other disadvantages include limited soft tissue contrast, higher cost, higher radiation exposure, and suboptimal imaging quality from interfering artefacts created by metal objects.11, 12
Non-ionizing, real-time and low-cost ultrasound has the potential to emerge as a useful cross-sectional imaging tool in implant therapy.13, 14 It is by definition acoustic waves with frequencies at or above 20 kHz, that are coupled and transmitted by means of a conducive medium into the human body. Resulting images are based on acoustic waves that are reflected back to the transmitter as they encounter heterogeneous tissues. Depending on the so-called time-of-fight t, i.e. the time the sound travels from the transmitting ultrasound probe into the body and reflect back to the probe, the physical distance d is then computed as d = t · c, where c is the sound speed in tissue. A significant body of research has demonstrated the potential of ultrasound for measuring both hard and soft tissues around teeth.15–17 Ultrasound can be utilized not only during the treatment phase but also during the surgery and at follow-up visits. With flapless implant surgeries becoming more popular, instantaneous intraoperative evaluation of the drill bit location is crucial. In the recent years, peri-implantitis has been receiving considerable attention both in the clinical and academic circles.18, 19 It is estimated, that approximately 20% of implants have marginal bone loss beyond the accepted clinical threshold.20 Additionally, implants that are prone to developing peri-implantitis need periodic clinical and radiological monitoring for early intervention and treatment success.18, 19 Cross-sectional imaging that can be repeatedly used to monitor marginal bone level after implants are in function is, therefore, much needed.
Ultrasound has been applied in almost every field of medicine but has never got its foot into clinical dentistry. The major hurdles include challenges associated with developing high-resolution and small form factor ultrasound probes that can be used in the oral cavity.21 Recent technological advances have allowed for the fabrication of miniature-sized probes with superior image quality. In addition, quantitative ultrasound parameters, derived from interactions between the sound and the object(s) to be tested, have significantly augment ultrasound’s diagnostic value. Examples include the use of sound speed for evaluating bone density22, 23 and sound reflective intensity patterns for evaluating implant stability.24–26 There is a lack of information about the current developments in research and the potential clinical indications of ultrasound technology in implant dentistry.15 Dissipating this information can potentially help encourage interested stakeholders and accelerate the commercialization of this useful imaging modality for the welfare of millions of patients. Therefore, the aim of this study is to systematically review available literature on the applications of ultrasound technology in the three phases, namely (1) pre-surgical planning phase, (2) intraoperative phase, and (3) post-surgical follow-up phase of implant therapy.
Methods and materials
A comprehensive literature search was initially run on 26 February 2017 and then rerun on 5 February 2018, and included several major health sciences databases, such as: Ovid Medline, Ovid Medline In-Process & Other Non-Indexed Citations, Ovid Medline Epub Ahead of Print, CINAHL (EBSCOhost), Embase.com, Dentistry and Oral Sciences Source (EBSCOhost), and Cochrane Central Register of Controlled Trials (Wiley). The search strategies combined dental implant and ultrasound imaging subject headings and keyword combinations. No restrictions were included in the searches. Duplicate citations were eliminated in Endnote X6 (Clarivate Analytics). The complete and reproducible search strategies are available in Addendum I.
Inclusion and exclusion criteria
All types of study designs involving the use of ultrasound imaging in implant dentistry were considered for inclusion, with no limitations based on age, gender, clinical setting (private practice vs educational institution or geographic location). Articles published prior to 1971 were excluded, because the earliest article on the use of ultrasound in dentistry was published in that year. Exclusion criteria included non-English studies, reviews and narrative summaries, and conference abstracts. Supplementary table 1lists the inclusion and exclusion criteria based on the objective of this review.
Study selection
Two authors (HL, VB) independently reviewed all titles and abstracts, as well as the full-text of articles that passed the initial review. Conflicts were resolved through discussion. Data extraction were done by two investigators (HL, VB) and abstracted in Table 1.
Table 1.
Listing and summary of included publications
| Author (year) | Study design | Objective | Ultrasound device (make & frequency) | Sample size | Methods | Results | Conclusions |
| Soft tissue evaluation | |||||||
| Traxler et al | Pre-clinical | Analyse the soft tissue covering the upper jaw and to compare the findings with mechanical methods | ATL, Ultramark 8 (10 MHz) | 8 cadaver heads/20 sites | The distance between the mucosal surface to bone was measured by ultrasound and directly and compared | Minimal deviations of 0.2 mm between ultrasound and direct measures | Sonographic imaging corresponds well to direct measurements |
| Culjat et al | Pre-clinical | Demonstrate a specialized ultrasound system in measuring the soft tissue thickness over implants | Prototype (16.1 MHz) | Two implants on one porcine model | Implants submerged in porcine ribs were measured by ultrasound by measuring the thickness of overlying soft tissue | Implants were located to an accuracy of 0.2 mm and soft tissue thickness was with a 0.5 mm error | The system was capable of measuring soft tissue thickness over bone and implants as well as locating implants |
| Culjat et al | Pre-clinical | Test an ultrasound system to measure the soft tissue thickness covering submerged implants | SP 7.5, Interson Corp (24 MHz) | Two implants on one porcine model | Implants placed in porcine bone samples were measured by three examiners recorded four ultrasound measurements | Ultrasound soft tissue measures were within 0.3 mm error | Ultrasound can be used to accurately detect dental implant fixtures and measure soft tissue |
| De Bryuckere et al | Pre-clinical | Use ultrasound to monitor soft tissue graft stability | EPOCH 600, Olympus, Aartselaar, Belgium (5MHz) | 37 subjects | Facial soft tissue thickness was measured before surgery, 2 week, 3 months and 1 year | Changes of 0.1 mm after 1 year | Grafted tissue stays stable at 1 year |
| Eghball et al | Pre-clinical | Assess validity and reproducibility of ultrasound for measuring mucosal thickness | EPOCH 600, Olympus, Aartselaar, Belgium (5MHz) | 4 human cadaver maxilla/100 sites | Mucosal thickness at 100 sites were measured with ultrasound and compared to micro-CT | Soft tissue thickness recorded with ultrasound and micro-CT had high correlation (r = 0.89) | The ultrasonic device can be non-invasive and reproducible to evaluate mucosal thickness |
| Hard tissue evaluation | |||||||
| Traxler et al | Clinical human | Compare residual ridge dimensions measured with ultrasound and open measurement | ATL, Ultramark 8 (10 MHz) | 4 patients/11 sites | Ridge width measured by ultrasound was compared with direct measurements | Ultrasound measures corresponds well to direct ridge mapping | Ultrasound for bone morphology evaluation is a valuable initial screening tool for implant treatment planning |
| Bertram et al | Clinical human | Assess reproducibility and validity of ultrasound peri-implant buccal bone loss | Sonoace Pico (12.5 MHz) | 25 patients/29 buccal bone defects | The distance between the upper thread of the implant to the most apical marginal bone was evaluated sonographically and compared to direct measures | Bone loss measurements made at moderate bone loss levels (3–6 mm) was most reliable | Ultrasound may be a reliable and valid method for assessing marginal bone loss and is defect depth dependent |
| Klein et al | Clinical human | (1) Assess alveolar crest UTV values (2) to compare UTV of osteoporosis and H/N radiation patients to health patients | DBMSonic 1200 instrument, IGEA (1.2 MHz) | 87 patients/204 sites | UTV values were measured and compared among different anatomical sites and patient groups | Significantly higher UTV in the max ant & mand post regions UTV corresponds clinically and histologically. UTV in osteoporotic patients were generally lower than in healthy patients |
UTV might identify critical bone quality before or to monitor bone healing after augmentation procedures |
| Salmon and Le Denmat | Clinical human | Present intraoral sonographic images generated by a novel ultrasound system | Prototype (25 MHz) | 3 patients/162 sites | All teeth of three subjects were evaluated on buccal and lingual sides by two examiners with ultrasound | Crestal bone and the marginal gingival levels were detectable at least 90% of the sites | This promising device requires large-scale clinical studies to determine whether it should remain a research tool or be used as a diagnostic tool for daily dental practice |
| Krammerer et al | Pre-clinical | Collerate UTV to histomorphometry and 3D-radiology | Modified DBMSonic 1200 instrument (1.2-MHz) | Six porcine rib samples (cortical, cancellous and mixed bone types) | Clinical cortical, cancellous, and mixed bone was measured with ultrasound, CBCT, micro-CT, and histomorphometry and compared | Statistically significant correlation (p < 0.001) was found between UTV, histomorphometry and radiogrpahic measures of bone parameters | UTV is able to discriminate between different bone types ex vivo |
| Degen et al | Pre-clinical | Analyse ultrasound for measuring the cortical bone thickness | Combination of low (5 MHz) and high (50 MHz) ultrasound system | 10 bovine rib blocks/ 10 implants (3.8 mm by 11 mm) | Dental implants were investigated using ultrasound, CBCT, and stereomicroscopy to measure the cortical bone thickness | The median deviation of ultrasound was 0.23 mm. CBCT method was slightly more accurate (median percent deviation of 9.2%) than the ultrasound method (10.3%) |
Ultrasound showed a high potential to supplement CBCT for measurements of the cortical bone thickness |
| Chan et al | Pre-clinical | Evaluate ultrasound to measure facial crestal bone level and thickness | Zonare ZS 3 (14 MHz) | 6 cadaver head/ 139 teeth | Crestal bone level/thickness of midfacial site were measured with ultrasound | Ultrasound bone level/thickness correlated well (0.8–0.9) with CBCT and direct measures | Ultrasound holds promise for evaluating crestal bone level/thickness |
| Vital structure evaluation | |||||||
| Lustig et al | Clinical human | Characterize lingual foramen artery using an ultrasound system | A.T.L. HD 3000 (10 MHz) | 20 patients | Blood vessel to the lingual foreman was identified and characterized by ultrasound | The diameter of the artery was 0.18–1.8 mm and the blood flow from 0.7 to 3.7 ml min-1 | Ultrasound is a reliable tool to visualize and measure the blood supply to the bony chin |
| Machtei et al | Clinical human | Identify IAC/maxillary sinus floor using an ultrasound device | JetGuide prototype | 14 patients; 21 implants (11 mandibular, 10 maxillary) | IAC and maxillary sinus was measured with ultrasound and compared to panoramic radiograph | A very strong positive correlation was observed between the two measurements in mandibles (r = 0.967; p = 0.0001). The correlation with respect to the floor of the sinus were weak |
The results support the value of this ultrasonic system in measuring the residual osseous depth |
| Rosenberg et al | Pre-clinical/clinical human | Measure hard tissue boundaries with ultrasound | JetGuide prototype (5 MHz) | (1) A cubic phantom (2) fresh porcine femora (3) nine patients | Bone boundaries of the three models were measured with ultrasound and compared to radiographic and direct measures | Ultrasound could differentiate the cortical bone from cancellous bone in both pre-clinical and clinical evaluations | Ultrasound technology can be employed as a useful tool to monitor intraosseous drilling |
| Zigdon- Giladi et al | Clinical human | Identify IAC with ultrasound | JetGuide prototype | 10 patients; 18 implant in mand | The distance between the bottom of the osteotome to the IAC was assessed using the ultrasound device and compared with standard panoramic radiographs | The mean difference by ultrasound and PAN was 0.18 mm (r = 0.61). That between ultrasound and CBCT was 0.21 mm |
The tested ultrasound device identifies the IAC |
| Chan et al | Pre-clinical | Evaluate ultrasound in measuring facial crestal bone level and thickness on different tooth types | Zonare ZS3 (14 MHz) | 6 cadaver heads, 10 sites in each head | (1)Greater palatine foramen, (2) lingual nerve (3) mental foramen were assessed with ultrasound. The images from ultrasound was compared to those obtained from CBT and /or direct measurements | The correlations were between 0.78 and 0.88. The mean absolute differences in crestal bone height and thickness were 0.09 mm | Proof-of-concept evidence that ultrasound can be a real time and non-invasive alternative |
| Implant stability evaluation | |||||||
| Veltri et al | Pre-clinical | Correlate amplitude-dependent speed of sound (ad-SOS) to implant insertion torque | DBMSonic 1200, IGEA (1.2 MHz) | 16 rabbits/2 anatomical sites, 32 implants/28 sites | Amplitude-dependent speed of sound (ad- SOS) of diaphysis (Group 1) and epiphyses (Group 2) of rabbit femurs and the insertion torque was measured and correlated | A negative correlation between insertion torque and ad-SOS (r = −0.7) | Ultrasound could convey potentially useful information on bone mechanical characteristics |
| Mathieu et al | Pre-clinical/ simulation | Study propagation of ultrasonic waves in cylinder implant prototypes and the sensitivity of these waves to the surrounding bone biomechanical properties | V129SM, Panametrics (10 MHz) | 40 rabbit femurs/4 conditions | Specific geometric configuration in four groups with a controlled amount of implant-bone contact were tested with ultrasound | A change of 1 mm of bone in contact with the implant, 1.1 mm of cortical bone thickness or 12% of trabecular bone mass density could be detectable by ultrasound | The ultrasound quantitative parameter extracted from the radiofrequency signals is sensitive to implant stability |
| Mathieu et al | Pre-clinical | Test ultrasound to measure the amount of bone in contact with implants | V129SM, Panametrics (10 MHz) | 10 implants in rabbit femurs | Four distinct clinical conditions corresponding to the amount of bone around the implant were tested with ultrasound | The Indicator I was significantly associated with the amount of bone in contact with the implants | The first step towards successful use of ultrasound to monitor dental implant stability |
| Ossi et al | Pre-clinical | Investigate the feasibility of monitoring implant primary stability using ultrasound | PAC Micro-80D AE sensor (0.1–1 MHz) | 40 implants (2 conditions) on 10 bovine ribs | Tight- and loose- fitting implants were measured by ultrasound and compared | Implants with good primary stability had a higher acoustic emission energy than shorter narrower implants | A simple transmission test, properly calibrated, should be able to assess the quality of bone-implant contact in the clinical situation |
| Kumar et al | Pre-clinical | Compare UTV bone quality measurements and correlate to RFA and POT | Modified DBMSonic 1200, IGEA (1.2 MHz) | Three porcine bone block types/nine implants | Porcine three bone types (1) cortical, (2) cancellous, (3) mix of cortical and cancellous bone were measured using UTV. RFA and POT were measured and compared to UTV | Higher values of RFA and POT were seen with higher UTV values (corresponding to higher bone quality) | A high correlation between UTV values and primary implant stability in ex vivo bone samples |
| Ossi et al | Pre-clinical | Assess ultrasound on various dental materials and bovine rib bones with various degrees of hydration | PAC Micro-80D AE sensor (0.1–1 MHz) | Materials used: bovine bone, GIC, Plaster of Paris, acrylic orthodontic resin, Type 4 dental stone | Fresh bovine rib, Plaster of Paris, acrylic orthodontic resin, GIC and Type 4 dental stones were used to study the axial surface transmission of AE | Ultrasound transmission through GIC is closest to the bone. AE energy through bone was found to be dependent on its degree of hydration |
These findings may have implications not only for AE transmission testing of bone- implant interfaces but also for passive AE monitoring of implants |
| Vayron et al | Pre-clinical | Use ultrasound system to study the response of an implant embedded in TSBC subjected to fatigue stresses | V129SM, Panametrics (10 MHz) | Seven implants in TSBC | Implants were embedded in TSBC. Indicator I, based on the temporal variation of the signal amplitude, was derived and its variation as a function of fatigue time was determined | No significant variation of indicator I as a function of time without mechanical solicitation. The indicator significantly increases as a function of fatigue time |
Ultrasound has the potential to emerge as a diagnostic tool to investigate the material properties around dental implants and help assess implant stability |
| Vayron et al | Pre-clinical | Use ultrasound to detect the amount of bone in contact with implants | Sonaxis, Besancon (10 MHz) | 10 implants placed in bovine humeral bone | Indicator I was determined for (1) implant hung in air was first measured, (2) after implantation and (3) after unscrewing the implant to reduce the contact area | Implant stability was measured by indicator I, calculated based on the amplitude of ultrasound signal after each turn. A significant association was found betweenIand the bone implant contact | The results indicates the feasibility of quantitative ultrasound techniques to assess implant primary stability in vitro |
| Vayron et al | Pre-clinical | Investigate the sensitivity of ultrasound response to bone healing around implants in vivo | V129SM, Panametrics (10 MHz) | 21 implants placed in femur of 7 rabbits | Indicator I was measured at insertion and at 2, 6 and 11 week healing and correlated to histological bone-implant contact ratio | Indicator I as a function of the healing time was between 7 and 40%. A statistically significant correlation between indicator I and BIC | Pave the way for the development of a new QUS method in dental implant therapy |
| Vayron et al | Simulation | Provide a model of ultrasound wave propagation through prototype titanium cylindrical implants | V129SM, Panametrics (10 MHz) | None (numerical analysis) | Different geometrical configurations were modelled to project various bone–implant interface situations and indicator I were measured | The implant ultrasonic response changes significantly when there is a liquid layer at implant surface | There is a potential of QUS techniques to study dental implant stability |
| Vayron et al | Simulation | Provide understanding of the ultrasound wave propagation in commercial dental implants | V129SM, Panametrics (10 MHz) | None (numerical analysis) | Three-dimensional finite element model was used to compute different geometrical configurations and related to indicator I | Indicator I decrease when bone quality increases, consistent with the experimental results | There is a potential of QUS techniques to study dental implant stability |
ad-SOS , amplitude-dependent speed of sound; AE, acoustic emission; BIC, bone-implant contact; CBCT, cone beam CT; GIC, glass-ionomer cement; H/N, Head & Neck; IAC, inferior alveolar canal; Mand, Mandibular; Max, Maxillary; POT, push-out test; QUS, quantitative ultrasound; RFA, radio frequency analysis; TSBC, tricalcium silicate-basedcement; UTV, ultrasound velocity.
Results
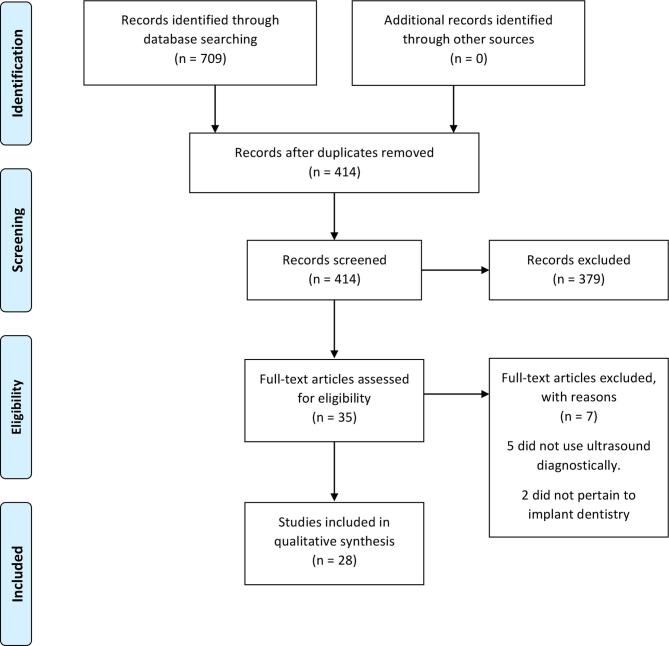
The initial search strategy retrieved 414 unique citations. After initial screening of titles and abstracts, as described in the "Materials and Methods" section, 35 articles were selected for full-text assessment. Of these, seven were excluded; the reasons for exclusion were summarized in Supplementary table 2. The remaining 28 articles13,14,22–47 were selected for inclusion in this review. See PRISMA flowchart for a breakdown of the screening process (Figure 1). The included articles have been summarized in Table 2 based on their clinical indications and study types (pre-clinical vs clinical study design). Four main potential indications were identified and will be described narratively below: evaluations of soft tissue, hard tissue, vital structure, and implant stability. For the first three indications, there have been a mixture of pre-clinical and human clinical studies available, indicating that the technology development is closer to clinical use. However, for studies aiming at evaluating implant stability, a significant portion of research is still in the pre-clinical stage.
Figure 1.
PRISMA flow chart for the systematic review.
Table 2.
Summary of the studies classified by the main indications and study designs
| Indication category | Specific parameter to measure | Author (year) | Study design | |
| Pre-clinical/simulation | Clinical human | |||
| Soft tissue evaluation | Tissue thickness | Traxler et al (1991) | V | |
| Culjat et al (2008) | V | |||
| Culjat et al (2012) | V | |||
| De Bruyckere et al (2015) | V | |||
| Eghbali et al (2016) | V | V | ||
| Hard tissue evaluation | Ridge width | Traxler et al (1992) | V | |
| Peri-implant bone level | Bertram et al (2007) | V | ||
| Bone density | Klein et al (2008) | V | ||
| Crestal bone level | Salmon et al (2011) | V | ||
| Bone density | Kammeler et al (2013) | V | ||
| Cortical bone thickness | Degen et al (2017) | V | ||
| Crestal bone level and thickness | Chan et al (2017) | V | ||
| Vital structure evaluation | Sublingual A. | Lustig et al (2003) | V | |
| Inferior alveolar canal & maxillary sinus | Machtei et al (2010) | V | ||
| Bone boundaries | Rosenberg et al (2014) | V | V | |
| Inferior alveolar canal | Zigdon- Giladi et al (2015) | V | ||
| Greater palatine foreman, mental foramen and lingual n. | Chan et al (2017) | V | ||
| Implant stability evaluation | Transmission sound velocity | Veltri et al (2010) | V | |
| Kumar et al (2012) | V | |||
| Transmission sound energy | Ossi et al (2011) | V | ||
| Ossi et al (2013) | V | |||
| Reflection sound amplitude pattern | Mathieu-a et al (2011) | V | ||
| Mathieu-b et al (2011) | V | |||
| Vayron et al (2013) | V | |||
| Vayron et al (2014a) | V | |||
| Vayron et al (2014b) | V | |||
| Vayron et al (2015) | V | |||
| Vayron et al (2016) | V | |||
Main indication 1: soft tissue evaluation
Several studies28, 29,32,40 evaluated the accuracy of ultrasound in measuring soft tissue thickness for implant planning. The mean difference between ultrasound and direct soft tissue thickness readings was 0.13–0.5 mm. The larger deviation is related to thicker tissue (approximately 5 mm). One study32 did a correlation analysis and found a strong correlation (r = 0.89) between ultrasound and direct measurements. Two studies30, 32 applied ultrasound to measure soft-tissue dimensional changes after a grafting procedure around implants in humans and found a reduction of 0.1 to 0.15 mm at 1-year follow-up.
Main indication 2: hard tissue evaluation
A descriptive study39 suggested the crestal bone level was detectable in at least 90% of the 162 studied sites from three patients on ultrasound images. A validation study13 on human cadavers of 139 teeth showed an accurate estimation of midfacial crestal bone height and thickness. The mean absolute differences in crestal bone height and thickness between ultrasound and CBCT were 0.09 mm [95% confidence interval (−1.20 to 1.00 mm)] and 0.03 mm [95% confidence interval (−0.48 to 0.54 mm)], respectively. Residual ridge width, measured with ultrasound on 11 sites from 4 patients, produced nearly (no statistics were provided) the same data as ridge mapping.41
Peri-implant bone level of 29 implants diagnosed with peri-implantitis was evaluated using ultrasonic imaging.27 Ultrasound measurements made at moderate bone loss levels (3–6 mm) were the most reliable (intraclass correlation coefficient = 0.81 for reproducibility and 0.76 for accuracy). However, the correlations in normal (<3 mm) and advanced bone loss (>6 mm) cases were moderate to poor (intraclass correlation coefficient = 0.63–0.73).
Jawbone density was possible to be evaluated using ultrasound.23 Significantly high ultrasound transmission velocity (UTV) was found in maxillary anterior and mandibular posterior regions than in maxillary posterior regions. A subsequent study by the same group22 found a high correlation (r > 0.9) between UTV values and those from histomorphometry and radiography.
Cortical bone thickness was determined with a combination of low (5 MHz) and high (50 MHz) frequency ultrasound set up, with approximately 10% deviation from the true thickness.31 Therefore, the authors concluded that ultrasound has a high potential to supplement CBCT in measurement of cortical bone thickness.
Main indication 3: vital structure evaluation
The inferior alveolar canal and the maxillary sinus floor were evaluated.35 Overall the differences between ultrasound and radiography measurements were minor (0.4 mm), with positive correlation (r = 0.57). After stratifying the data, the differences in inferior alveolar canal readings were 0.1 mm, with high correlation (0.967). Subsequently, a follow-up study with a larger sample size was conducted by the same group, showing the mean differences in residual bone height were 0.18 mm, with good correlation (r = 0.61).47
The diameter, direction of blood flow, and blood volume of the sublingual artery were evaluated.34 The average diameter of the artery was 1.41 ± 0.34 mm and the average blood flow 2.92 ± 3.19 ml min-1.
Bone boundaries should not be violated during implant surgery, otherwise soft tissue damage and surgical complications may occur. A study38 measured bone boundaries with two parameters: the depth of drill penetration into bone (drilled tract), and the distance between the drill tip to the bone boundary (residual depth). The correlations of ultrasound and mechanical measurements in the pre-clinical settings were ~0.99, with the mean measurement differences between 0.27 and 1.1 mm. In the clinical settings, the correlation between ultrasound and mechanical measurements was 0.78, with the mean difference of 0.05 mm. Therefore, the study concluded this ultrasound method could be useful to monitor intraosseous drilling.
In a proof-of-principle study,14 the following three anatomical structures were identified with ultrasound: the greater palatine foramen, the mental foramen and the lingual nerve. The three structures were clearly shown; in addition, merged ultrasound and CBCT images demonstrated overall spatial accuracy of ultrasound images.
Main indication 4: implant–bone interface evaluation
Studies using “transmission sound velocity” as an indicator
In an ex vivo rabbit cadaver study,46 the correlation of a quantitative ultrasound parameter, amplitude-dependent speed of sound to implant insertion torque was studied. A significant negative correlation (−0.706) was found, implying that samples with a lower amplitude-dependent speed of sound measurement have a higher implant insertion torque.
An ex vivo porcine cadaver study33 evaluated the “UTV” on implant primary stability, measured by radiofrequency analysis and push-out test. Samples with a higher UTV also have higher radio frequency analysis and push-out test values, indicating that this ultrasound method can give reasonably objective information on the expected primary implant stability.
Studies using “transmission sound amplitude (energy)” as an indicator
An ex vivo bovine cadaver study36 investigated the feasibility of monitoring implant primary stability using a simple transmission ultrasound test. Higher implant primary stability was associated with higher received amplitudes. The same group37 also evaluated variables that may affect ultrasound energy readings. The results showed transmission through glass–ionomer cement to be closest to human bone. The study also showed ultrasound transmission through bone is dependent on its degree of hydration.
Studies using “reflection sound amplitude pattern” as an indicator
A single research group contributed to the literature in this field; therefore, the sequence in this section has been organized based on the study design.
Ex-vivo studies/material testing
The potential of ultrasound to measure implant–bone contact was evaluated.25 The radiofrequency (rf) signal during the first 60 μs was recorded and the summation of the maximum amplitude of each echo was calculated as a quantitative indicator I. The results showed that the indicatorIwas significantly associated with the amount of implant–bone contact. Subsequently, a significant association (p < 10−5) between the amount of implant–bone contact and indicatorIwas found.
Ultrasound was used to test the fatigue behaviour of a bone substitute, tricalcium silicate-based cement around dental implants.26 The output parameter significantly increased as a function of fatigue time. This increase may be due to the degradation of the material at the implant interface.
Numerical simulation studies
A computer-based numerical simulation study was performed in order to understand ultrasound wave propagation in cylindrical implants.24ant, or 1.1 mm of cortical thickness or 12% of trabecular bone mass density could be detected by ultrasound. Three types of ultrasonic waves during propagation were identified, direct, transverse and lateral waves. The results also showed that, either a change of 1 mm of bone in contact with the implant, or 1.1 mm of cortical thickness or 12% of trabecular bone mass density could be detected by ultrasound.
More clinically relevant modelling of the implant–bone system for studying ultrasonic wave propagation was performed with a three-dimensional axisymmetric geometrical configuration model.43 The implant ultrasound response changes significantly when a liquid layer was located at the implant interface. Furthermore, the indicator I decreases as a function of healing time. Subsequently, the geometry of a commercially available implant was modelled in lieu of a cylindrical shaped implant used in previous studies.44 The simulated ultrasound response was also consistent with the experimental results.
In-vivo preclinical study
A pre-clinical study using a rabbit model45 was performed to investigate the sensitivity of the ultrasound to implant–bone contacts. Ultrasound response varied as a function of healing time between 7 and 40%. The results also showed that ultrasound correlated (R2 = 0.45) with the implant–bone contact.
Discussion
This systematic review successfully identified 28 manuscripts that studied the applications of ultrasound for use in implant therapy in three treatment phases. Table 3 summarizes the potential indications of using ultrasound in these phases.
Table 3.
Potential clinical indications of ultrasonography for different phases of implant therapy
| Treatment phase | Potential indications |
| Planning phase | Identify vital structures |
| Evaluate soft and hard tissue biotype | |
| Evaluate ridge width | |
| Indicate bone density | |
| Evaluate cortical bone thickness | |
| Surgical phase | Identify vital structures |
| Evaluate drill bit-bone boundary distances | |
| Indicate primary stability | |
| Follow-up phase | Evaluate marginal bone level around implants |
| Indicate implant–bone stability |
During each treatment phase, accurate knowledge of soft/hard tissue dimensions, relationship to vital structures and bone density measurement are pre-requisites for safe and successful implant placement. Additionally, tissue biotype has many clinical implications, e.g. the amount of marginal bone remodelling, timing of implant placement, and selection of restorations, etc.48 Various methods have been developed to evaluate soft tissue biotype, including visual estimation and probing techniques but with limitations.49, 50 Ultrasound is an excellent tool for tissue biotype evaluation, with a measurement deviation less than 10%.28–30,40 Ridge width before surgery can currently only be revealed with CBCT. Preclinical data suggested ultrasound can image ridge surface topography and therefore measure ridge width.41 It is possible ultrasound can become a chairside initial screening device for measuring ridge width. Ultrasound has been used to diagnose and follow-up osteoporosis clinically. The velocity of ultrasound in isotropic materials correlates to the modulus of elasticity and the physical density. Although bone is anisotropic, ultrasound velocity measures have been proved an indirect indicator of bone elasticity and density. Two identified studies22, 23 using a similar mechanism to indicate alveolar bone density, suggesting that ultrasound holds a potential to quantify ridge density. The greater palatine foramen, mandibular lingual foramen and lingual nerve are hard to identify with the current clinical methods. Ultrasound would be useful to identify and characterize these important structures.14, 34 Therefore, ultrasound could potentially find its unique role to provide important clinical information during the treatment planning phase.
During the surgery, it is fundamental to place an implant in an ideal position without disrupting nearby vital structures, such as bone boundaries, the inferior alveolar canal, and the maxillary sinus, etc. Currently, this is done with a passive surgical guide that is made from a dental model or from CBCT images. Intraoperative drill bit location is confirmed with intraoral radiographs. Ultrasound can detect the impedance differences between the cancellous bone and the cortical bone that surrounds important structures;35, 38,47 therefore, it could be used in lieu of radiographs to avoid surgical complications. Flapless implant surgery is becoming more popular because of tissue preservation, faster healing and reduced morbidity.51–53 Cross-sectional drill-bit location could be imaged in real-time with ultrasound to provide surgical feedback.
During the healing and maintenance phase, monitoring marginal bone level and implant stability is required to secure implant success. Marginal bone loss is the hallmark of peri-implantitis, which is estimated to occur in 20% of cases.20 Bertram et al27 were among the pioneers, if not the first to image peri-implant bone level in humans, using 12.5 MHz linear array ultrasound probe. At this frequency, ultrasound has already shown a potential to diagnose bone loss; higher frequency can improve image resolution and possible diagnostic value. Currently, implant stability is evaluated with Periotest® and OsstellTM. Periotest measures the contact time to the tested implant; whereas OsstellTM is to use resonance frequency for measuring implant stability.54 Quantitative ultrasound parameters, like transmission sound speed/energy and reflection sound amplitude pattern, have been used to study implant stability. Experimental and simulation studies identified in this manuscript suggested these parameters were correlated with the degree of implant-bone contact. However, due to complexity of sound wave propagation in implants and bone, it will take more development and research before we can see their clinical uses.
Ultrasound limitations and disadvantages
Limitations of ultrasound include the need of a medium for sound conduction, inability to penetrate into bone, and narrow field of view. Acoustic gel is needed to apply on mucosal surface for ultrasound imaging. Ultrasound can image bone surfaces but not inside bone. Therefore, ultrasound is unable to diagnose hard tissue pathology or intraosseous structures. Ultrasound is able to image a focused site in 2D but not the entire jawbone. Therefore, it is best indicated for single implant restoration. Three-dimensional ultrasound images would be desirable. They can be achieved by mechanically moving a one-dimensional array probe, following by imaging reconstruction, or by use of a 2D array probe. Additionally, since ultrasound imaging is new to dentistry, a learning curve to adapt to this technology is required.
Future research directions
Anatomical imaging is only suitable for measuring static tissue dimensions of interest. A new ultrasound-based imaging modality, photoacoustic imaging, may be useful to differentiate minute changes in water content, ratio of oxygenated/deoxygenated hemoglobin and blood volume in soft tissues. Future research should focus on using photoacoustic imaging for evaluating implant wound healing and peri-implantitis activity.
Future clinical directions
Although ultrasound imaging is currently not used in dental clinics, there are continued interests in introducing this imaging modality to clinical dentistry. For the first time, this systematic review successfully identified many potential indications for ultrasound use in implant therapy. Interested stakeholders, e.g. researchers, dentists, ultrasound manufacturers, and entrepreneurs are encouraged to collaborate to bring ultrasound into clinics for the welfare of millions of patients.
REFERENCES
- 1.Brånemark PI, Hansson BO, Adell R, Breine U, Lindström J, Hallén O, et al. . Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl 1977; 16: 1–132. [PubMed] [Google Scholar]
- 2.Lambrecht JT, Filippi A, Künzel AR, Schiel HJ. Long-term evaluation of submerged and nonsubmerged ITI solid-screw titanium implants: a 10-year life table analysis of 468 implants. Int J Oral Maxillofac Implants 2003; 18: 826–34. [PubMed] [Google Scholar]
- 3.Vandenberghe B, Jacobs R, Bosmans H. Modern dental imaging: a review of the current technology and clinical applications in dental practice. Eur Radiol 2010; 20: 2637–55. doi: 10.1007/s00330-010-1836-1 [DOI] [PubMed] [Google Scholar]
- 4.Correa LR, Spin-Neto R, Stavropoulos A, Schropp L, da Silveira HE, Wenzel A. Planning of dental implant size with digital panoramic radiographs, CBCT-generated panoramic images, and CBCT cross-sectional images. Clin Oral Implants Res 2014; 25: 690–5. doi: 10.1111/clr.12126 [DOI] [PubMed] [Google Scholar]
- 5.Chan HL, Misch K, Wang HL. Dental imaging in implant treatment planning. Implant Dent 2010; 19: 288–98. doi: 10.1097/ID.0b013e3181e59ebd [DOI] [PubMed] [Google Scholar]
- 6.Tyndall DA, Price JB, Tetradis S, Ganz SD, Hildebolt C, Scarfe WC, et al. . Position statement of the American Academy of Oral and Maxillofacial Radiology on selection criteria for the use of radiology in dental implantology with emphasis on cone beam computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 113: 817–26. doi: 10.1016/j.oooo.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 7.Dagassan-Berndt DC, Zitzmann NU, Walter C, Schulze RK. Implant treatment planning regarding augmentation procedures: panoramic radiographs vs. cone beam computed tomography images. Clin Oral Implants Res 2016; 27: 1010–6. doi: 10.1111/clr.12666 [DOI] [PubMed] [Google Scholar]
- 8.Deeb G, Antonos L, Tack S, Carrico C, Laskin D, Deeb JG. Is cone-beam computed tomography always necessary for dental implant placement? J Oral Maxillofac Surg 2017; 75: 285–9. doi: 10.1016/j.joms.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 9.Rios HF, Borgnakke WS, Benavides E. The use of cone-beam computed tomography in management of patients requiring dental implants: an American Academy of Periodontology best evidence review. J Periodontol 2017; 88: 946–59. doi: 10.1902/jop.2017.160548 [DOI] [PubMed] [Google Scholar]
- 10.Joda T, Brägger U, vs D. Digital vs. conventional implant prosthetic workflows: a cost/time analysis. Clin Oral Implants Res 2015; 26: 1430–5. doi: 10.1111/clr.12476 [DOI] [PubMed] [Google Scholar]
- 11.Benavides E, Rios HF, Ganz SD, An CH, Resnik R, Reardon GT, et al. . Use of cone beam computed tomography in implant dentistry: the International Congress of Oral Implantologists consensus report. Implant Dent 2012; 21: 78–86. doi: 10.1097/ID.0b013e31824885b5 [DOI] [PubMed] [Google Scholar]
- 12.Mandelaris GA, Scheyer ET, Evans M, Kim D, McAllister B, Nevins ML, et al. . American Academy of Periodontology best evidence consensus statement on selected oral applications for cone-beam computed tomography. J Periodontol 2017; 88: 939–45. doi: 10.1902/jop.2017.170234 [DOI] [PubMed] [Google Scholar]
- 13.Chan HL, Sinjab K, Chung MP, Chiang YC, Wang HL, Giannobile WV, et al. . Non-invasive evaluation of facial crestal bone with ultrasonography. PLoS One 2017; 12: e0171237. doi: 10.1371/journal.pone.0171237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan HL, Wang HL, Fowlkes JB, Giannobile WV, Kripfgans OD. Non-ionizing real-time ultrasonography in implant and oral surgery: A feasibility study. Clin Oral Implants Res 2017; 28: 341–7. doi: 10.1111/clr.12805 [DOI] [PubMed] [Google Scholar]
- 15.Marotti J, Heger S, Tinschert J, Tortamano P, Chuembou F, Radermacher K, et al. . Recent advances of ultrasound imaging in dentistry-a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 115: 819–32. doi: 10.1016/j.oooo.2013.03.012 [DOI] [PubMed] [Google Scholar]
- 16.Nguyen KC, Le LH, Kaipatur NR, Major PW. Imaging the cemento-enamel junction using a 20-MHz ultrasoni transducer. Ultrasound Med Biol 2016; 42: 333–8. doi: 10.1016/j.ultrasmedbio.2015.09.012 [DOI] [PubMed] [Google Scholar]
- 17.Nguyen KT, Le LH, Kaipatur NR, Zheng R, Lou EH, Major PW. High-resolution ultrasonic imaging of dento-periodontal tissues using a multi-element phased array system. Ann Biomed Eng 2016; 44: 2874–86. doi: 10.1007/s10439-016-1634-2 [DOI] [PubMed] [Google Scholar]
- 18.Chan HL, Lin GH, Suarez F, MacEachern M, Wang HL. Surgical management of peri-implantitis: a systematic review and meta-analysis of treatment outcomes. J Periodontol 2014; 85: 1027–41. doi: 10.1902/jop.2013.130563 [DOI] [PubMed] [Google Scholar]
- 19.Khoshkam V, Chan HL, Lin GH, MacEachern MP, Monje A, Suarez F, et al. . Reconstructive procedures for treating peri-implantitis: a systematic review. J Dent Res 2013; 92(12 Suppl): 131S–8. doi: 10.1177/0022034513509279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol 2015; 42(Suppl 16): S158–S171. doi: 10.1111/jcpe.12334 [DOI] [PubMed] [Google Scholar]
- 21.Ghorayeb SR, Bertoncini CA, Hinders MK. Ultrasonography in dentistry. IEEE Trans Ultrason Ferroelectr Freq Control 2008; 55: 1256–66. doi: 10.1109/TUFFC.2008.788 [DOI] [PubMed] [Google Scholar]
- 22.Kämmerer PW, Kumar VV, Brüllmann D, Götz H, Kann PH, Al-Nawas B, et al. . Evaluation of ultrasound transmission velocity and 3-dimensional radiology in different bone types for dental implantology: a comparative ex vivo study. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 116: e77–e84. doi: 10.1016/j.oooo.2011.11.028 [DOI] [PubMed] [Google Scholar]
- 23.Klein MO, Grötz KA, Manefeld B, Kann PH, Al-Nawas B. Ultrasound transmission velocity for noninvasive evaluation of jaw bone quality in vivo before dental implantation. Ultrasound Med Biol 2008; 34: 1966–71. doi: 10.1016/j.ultrasmedbio.2008.04.016 [DOI] [PubMed] [Google Scholar]
- 24.Mathieu V, Anagnostou F, Soffer E, Haiat G. Numerical simulation of ultrasonic wave propagation for the evaluation of dental implant biomechanical stability. J Acoust Soc Am 2011; 129: 4062–72. doi: 10.1121/1.3586788 [DOI] [PubMed] [Google Scholar]
- 25.Mathieu V, Anagnostou F, Soffer E, Haïat G. Ultrasonic evaluation of dental implant biomechanical stability: an in vitro study. Ultrasound Med Biol 2011; 37: 262–70. doi: 10.1016/j.ultrasmedbio.2010.10.008 [DOI] [PubMed] [Google Scholar]
- 26.Vayron R, Karasinski P, Mathieu V, Michel A, Loriot D, Richard G, et al. . Variation of the ultrasonic response of a dental implant embedded in tricalcium silicate-based cement under cyclic loading. J Biomech 2013; 46: 1162–8. doi: 10.1016/j.jbiomech.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 27.Bertram S, Emshoff R. Sonography of periimplant buccal bone defects in periodontitis patients: A pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 105: 99–103. doi: 10.1016/j.tripleo.2007.01.014 [DOI] [PubMed] [Google Scholar]
- 28.Culjat MO, Choi M, Singh RS, Grundfest WS, Brown ER, White SN. Ultrasound detection of submerged dental implants through soft tissue in a porcine model. J Prosthet Dent 2008; 99: 218–24. doi: 10.1016/S0022-3913(08)60046-3 [DOI] [PubMed] [Google Scholar]
- 29.Culjat MO, Choi M, Singh RS, White SN. Ultrasound imaging of dental implants. 2012 Conference proceedings: The British Institute of Radiology.; 2012. 456–9. [DOI] [PubMed] [Google Scholar]
- 30.De Bruyckere T, Eghbali A, Younes F, De Bruyn H, Cosyn J. Horizontal stability of connective tissue grafts at the buccal aspect of single implants: a 1-year prospective case series. J Clin Periodontol 2015; 42: 876–82. doi: 10.1111/jcpe.12448 [DOI] [PubMed] [Google Scholar]
- 31.Degen K, Habor D, Radermacher K, Heger S, Kern JS, Wolfart S, et al. . Assessment of cortical bone thickness using ultrasound. Clin Oral Implants Res 2017; 28: 520–8. doi: 10.1111/clr.12829 [DOI] [PubMed] [Google Scholar]
- 32.Eghbali A, De Bruyn H, Cosyn J, Kerckaert I, Van Hoof T. Ultrasonic assessment of mucosal thickness around implants: validity, reproducibility, and stability of connective tissue grafts at the buccal aspect. Clin Implant Dent Relat Res 2016; 18: 51–61. doi: 10.1111/cid.12245 [DOI] [PubMed] [Google Scholar]
- 33.Kumar VV, Sagheb K, Klein MO, Al-Nawas B, Kann PH, Kämmerer PW. Relation between bone quality values from ultrasound transmission velocity and implant stability parameters-an ex vivo study. Clin Oral Implants Res 2012; 23: 975–80. doi: 10.1111/j.1600-0501.2011.02250.x [DOI] [PubMed] [Google Scholar]
- 34.Lustig JP, London D, Dor BL, Yanko R. Ultrasound identification and quantitative measurement of blood supply to the anterior part of the mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003; 96: 625–9. doi: 10.1016/j.tripleo.2003.08.015 [DOI] [PubMed] [Google Scholar]
- 35.Machtei EE, Zigdon H, Levin L, Peled M. Novel ultrasonic device to measure the distance from the bottom of the osteotome to various anatomic landmarks. J Periodontol 2010; 81: 1051–5. doi: 10.1902/jop.2010.090621 [DOI] [PubMed] [Google Scholar]
- 36.Ossi Z, Abdou W, Reuben RL, Ibbetson RJ. In vitro assessment of bone-implant interface using an acoustic emission transmission test. Proc Inst Mech Eng H 2012; 226: 63–9. doi: 10.1177/0954411911428696 [DOI] [PubMed] [Google Scholar]
- 37.Ossi Z, Abdou W, Reuben RL, Ibbetson RJ. Transmission of acoustic emission in bones, implants and dental materials. Proc Inst Mech Eng H 2013; 227: 1237–45. doi: 10.1177/0954411913500204 [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg N, Craft A, Halevy-Politch J. Intraosseous monitoring and guiding by ultrasound: a feasibility study. Ultrasonics 2014; 54: 710–9. doi: 10.1016/j.ultras.2013.09.008 [DOI] [PubMed] [Google Scholar]
- 39.Salmon B, Le Denmat D. Intraoral ultrasonography: development of a specific high-frequency probe and clinical pilot study. Clin Oral Investig 2012; 16: 643–9. doi: 10.1007/s00784-011-0533-z [DOI] [PubMed] [Google Scholar]
- 40.Traxler M, Solar P, Ulm C, Gritzmann N. Ultrasonographic measurement of the soft-tissue of the upper jaw. Acta Radiol 1991; 32: 3–5. doi: 10.1177/028418519103200102 [DOI] [PubMed] [Google Scholar]
- 41.Traxler M, Ulm C, Solar P, Lill W. Sonographic measurement versus mapping for determination of residual ridge width. J Prosthet Dent 1992; 67: 358–61. doi: 10.1016/0022-3913(92)90246-7 [DOI] [PubMed] [Google Scholar]
- 42.Vayron R, Mathieu V, Michel A, Haïat G. Assessment of in vitro dental implant primary stability using an ultrasonic method. Ultrasound Med Biol 2014; 40: 2885–94. doi: 10.1016/j.ultrasmedbio.2014.03.035 [DOI] [PubMed] [Google Scholar]
- 43.Vayron R, Nguyen VH, Bosc R, Naili S, Haïat G. Finite element simulation of ultrasonic wave propagation in a dental implant for biomechanical stability assessment. Biomech Model Mechanobiol 2015; 14: 1021–32. doi: 10.1007/s10237-015-0651-7 [DOI] [PubMed] [Google Scholar]
- 44.Vayron R, Nguyen VH, Bosc R, Naili S, Haïat G. Assessment of the biomechanical stability of a dental implant with quantitative ultrasound: a three-dimensional finite element study. J Acoust Soc Am 2016; 139: 773–80. doi: 10.1121/1.4941452 [DOI] [PubMed] [Google Scholar]
- 45.Vayron R, Soffer E, Anagnostou F, Haïat G. Ultrasonic evaluation of dental implant osseointegration. J Biomech 2014; 47: 3562–8. doi: 10.1016/j.jbiomech.2014.07.011 [DOI] [PubMed] [Google Scholar]
- 46.Veltri M, Valenti R, Ceccarelli E, Balleri P, Nuti R, Ferrari M. The speed of sound correlates with implant insertion torque in rabbit bone: an in vitro experiment. Clin Oral Implants Res 2010; 21: 751–5. doi: 10.1111/j.1600-0501.2009.01873.x [DOI] [PubMed] [Google Scholar]
- 47.Zigdon-Giladi H, Saminsky M, Elimelech R, Machtei EE. Intraoperative measurement of the distance from the bottom of osteotomy to the mandibular canal using a novel ultrasonic device. Clin Implant Dent Relat Res 2016; 18: 1034–41. doi: 10.1111/cid.12362 [DOI] [PubMed] [Google Scholar]
- 48.Fu JH, Yeh CY, Chan HL, Tatarakis N, Leong DJ, Wang HL. Tissue biotype and its relation to the underlying bone morphology. J Periodontol 2010; 81: 569–74. doi: 10.1902/jop.2009.090591 [DOI] [PubMed] [Google Scholar]
- 49.De Rouck T, Collys K, Wyn I, Cosyn J. Instant provisionalization of immediate single-tooth implants is essential to optimize esthetic treatment outcome. Clin Oral Implants Res 2009; 20: 566–70. doi: 10.1111/j.1600-0501.2008.01674.x [DOI] [PubMed] [Google Scholar]
- 50.Kan JY, Morimoto T, Rungcharassaeng K, Roe P, Smith DH. Gingival biotype assessment in the esthetic zone: visual versus direct measurement. Int J Periodontics Restorative Dent 2010; 30: 237–43. [PubMed] [Google Scholar]
- 51.Bashutski JD, Wang HL, Rudek I, Moreno I, Koticha T, Oh TJ. Effect of flapless surgery on single-tooth implants in the esthetic zone: a randomized clinical trial. J Periodontol 2013; 84: 1747–54. doi: 10.1902/jop.2013.120575 [DOI] [PubMed] [Google Scholar]
- 52.Hsu YT, Chan HL, Rudek I, Bashutski J, Oh WS, Wang HL, et al. . Comparison of clinical and radiographic outcomes of platform-switched implants with a rough collar and platform-matched implants with a smooth collar: a 1-year randomized clinical trial. Int J Oral Maxillofac Implants 2016; 31: 382–90. doi: https://doi.org/10.11607/jomi.4189 [DOI] [PubMed] [Google Scholar]
- 53.Oh TJ, Shotwell J, Billy E, Byun HY, Wang HL. Flapless implant surgery in the esthetic region: advantages and precautions. Int J Periodontics Restorative Dent 2007; 27: 27–33. [PubMed] [Google Scholar]
- 54.Chan HL, El-Kholy K, Fu JH, Galindo-Moreno P, Wang HL. Implant primary stability determined by resonance frequency analysis in surgically created defects: a pilot cadaver study. Implant Dent 2010; 19: 509–19. doi: 10.1097/ID.0b013e3181fa7f6a [DOI] [PubMed] [Google Scholar]