Sickle cell disease (SCD) is an inherited red blood cell (RBC) disorder caused by homozygous or compound heterozygous mutations in the β-globin gene. The phenotype varies markedly and sex may contribute to this difference. Similar to the general population,[1] females with SCD appear to have a survival advantage;[2] the explanation for this difference is unclear. Increased hemolysis is associated with certain complications, such as leg ulcers, pulmonary hypertension, priapism, chronic kidney disease, and larger-artery ischemic stroke, and with an increased risk for early mortality in SCD.[2] Reduced hemolysis has been observed in RBC units from females versus males in the general population and lower hemolytic markers have been observed in females versus males with SCD.[3] Possible explanations for this difference in hemolysis include increased F cells in females versus males,[4] X-linked G6PD deficiency,[5] or the effects of sex hormones on RBC membrane stability.[3]
We aimed to characterize the sex-associated differences in the degree of hemolysis, hemolysis-related complications and survival patterns in 370 SCD adults from the University of Illinois at Chicago (UIC) cohort and 668 SCD patients from the multicenter Walk-PHaSST cohort. Protocols were approved by Institutional Review Boards. Subjects provided written informed consent prior to recruitment. Laboratory and clinical data were collected from steady-state, defined as a routine clinic visit without the patient having symptoms of a vaso-occlusive episode. Hemoglobin F% (HbF%) determinations were performed either prior to the institution of hydroxyurea therapy or at the time of the baseline visit if not on hydroxyurea therapy. Pulse pressure was defined as the difference between diastolic and systolic blood pressures; a cutoff of 60 mmHg was used as a marker of increased cardiovascular risk. Tricuspid regurgitant jet velocity (TRJV) was assessed from steady-state echocardiograms and elevated TRJV was defined as TRJV >3.0 m/s, an independent predictor for early mortality.[2]
Genome-wide expression of protein coding genes was carried out using an Affymetrix Human Exon 2.0 ST array in RNA isolated from the peripheral blood mononuclear cells of 180 SCD patients at UIC. X-linked G6PD status was determined by PCR in 360 UIC patients (217 females, 143 males) and in 485 Walk-PHaSST patients (263 females, 222 males) using the Taqman genotyping assay (Applied BioSystems, Foster City, CA, USA) following the manufacturer’s protocol. G6PD A- was defined as males hemizygous and females homozygous for both the G6PD202A and the G6PD376G alleles. G6PD A was defined as males hemizygous and females homozygous for the G6PD376G alleles and have at least one wild-type G6PD202G allele. G6PD B was defined as males and females who have at least one wild-type G6PD376A allele.
The relationship of HbF% and G6PD status with hemolytic markers was determined using linear regression analysis, adjusting for age, SCD genotype, and hydroxyurea therapy as well as by site in the Walk-PHaSST cohort. The relationships of sex with linear and categorical variables were determined using linear and logistic regression analysis, respectively, with similar adjustments in addition to HbF%. Interaction analysis for the effect of G6PD A- on the relationship between sex and hemolytic markers was performed using ANOVA. The ratio of females:males and hemolytic variables according to decile of age was analyzed using Cochran’s linear trend and the linear trend test, respectively. Statistical analyses were performed using Systat 11 (Systat Software Corporation, Chicago, IL, USA).
Baseline characteristics by sex are provided in Table 1. HbF% was higher in females versus males in both cohorts (P≤0.008). Higher HbF% was associated with lower AST, LDH, and absolute reticulocyte count and a higher hemoglobin concentration (P ≤ 0.02) in both cohorts (Supplementary Table1). Higher HbF% was associated with a lower total bilirubin concentration in the Walk-PHaSST cohort (P = 0.0008) and with a trend for an association with a lower total bilirubin concentration in the UIC cohort (P = 0.08). After adjusting for HbF%, females had lower AST, LDH, reticulocyte counts, and total bilirubin concentrations in both cohorts (Table 1).
Table1:
Demographic features and markers of hemolysis in the UIC and Walk-PHaSST cohorts.
| UIC | Walk-PHaSST | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||||
| N | Females | N | Males | P-value | N | Females | N | Males | P-value | |
| Age | 224 | 34 (25–45) | 146 | 28 (22–41) | 0.016 | 364 | 36 (25–47) | 304 | 33 (22–45) | 0.005 |
| HU Use | 224 | 98 (44%) | 146 | 66 (45%) | 0.8 | 364 | 115 (32%) | 304 | 105 (35%) | 0.4 |
| - SS/SB0 | 161 (72%) | 119 (82%) | 246 (73%) | 225 (78%) | ||||||
| ALT (U/L) | 223 | 22 (15 – 32) | 146 | 23 (17 – 31) | 0.6 | - | - | - | - | - |
| α-thalassemia | 220 | 80 (37%) | 137 | 41 (30%) | 0.3 | 302 | 99 (33%) | 259 | 83 (32%) | 0.9 |
| G6PD A- | 217 | 8 (4%) | 143 | 16 (11%) | 0.005 | 263 | 10 (4%) | 222 | 23 (10%) | 0.004 |
| HbF % | 188 | 4.0 (1.8–7.6) | 118 | 3.1 (1.3–6.1) | 0.008 | 182 | 3.0 (1.1–7.2) | 155 | 2.3 (1–5.5) | 0.003 |
| Markers of Hemolysis | ||||||||||
| AST (U/L) | 218 | 34 (25–47) | 145 | 39 (30–52) | 0.04 | 319 | 35 (25–49) | 280 | 41 (30–60) | <0.0001 |
| Hemoglobin (g/dL) | 224 | 8.9 (8.0 – 10.2) | 146 | 9.1 (8.1 – 10.6) | 0.2 | 321 | 9.1 (8.0 – 10.4) | 287 | 9.4 (8.2 – 11.0) | < 0.0001 |
| LDH (U/L) | 203 | 287 (212–396) | 132 | 342 (260–494) | <0.0001 | 305 | 351 (239–494) | 260 | 396 (277–636) | 0.01 |
| Retic count (x103 U/L) | 222 | 256 (171–373) | 146 | 333 (219–441) | 0.03 | 303 | 187 (127–281) | 272 | 232 (157–321) | 0.007 |
| Total Bilirubin (mg/dL) | 217 | 1.9 (1.3–3.2) | 145 | 2.5 (1.8–4.0) | 0.002 | 325 | 3.4 (2.1–5.5) | 285 | 4.4 (2.7–6.9) | 0.0005 |
| Hemolysis-Related Complications | ||||||||||
| CKD | 196 | 102 (52%) | 121 | 61 (50%) | 0.7 | 201 | 101 (50%) | 161 | 84 (52%) | 0.2 |
| Leg ulcers | 217 | 14 (6%) | 142 | 26 (18%) | 0.0004 | 364 | 65 (18%) | 304 | 61(20%) | 0.3 |
| Pulse pressure >60mmhg | 224 | 28 (13%) | 146 | 44 (30%) | <0.0001 | 334 | 57 (17%) | 289 | 79 (27%) | 0.0004 |
| Stroke | 224 | 48 (21%) | 146 | 29 (20%) | 0.4 | 334 | 22 (7%) | 289 | 28 (10%) | 0.2 |
| TRJV >3.0 m/s | 146 | 6 (4%) | 94 | 9 (10%) | 0.1 | 312 | 30 (10%) | 273 | 43 (16%) | 0.004 |
| Vaso-occlusive Complications | ||||||||||
| ACS | 186 | 117 (63%) | 118 | 64 (54%) | 0.2 | 364 | 192 (53%) | 304 | 188 (62%) | 0.05 |
| AVN | 186 | 70 (38%) | 118 | 42 (36%) | 0.8 | 364 | 113 (31%) | 304 | 88 (29%) | 0.7 |
| VOC ≥ 3/year | 163 | 95 (58%) | 108 | 67 (62%) | 0.5 | 364 | 143 (39%) | 304 | 106 (35%) | 0.06 |
Continuous variables are presented as median values (interquartile range). UIC: University of Illinois at Chicago; Walk-PHaSST: Walk-Treatment of Pulmonary Hypertension and Sickle Cell Disease with Sildenafil Therapy; HU: Hydroxyurea; G6PD: Glucose −6-Phosphate Dehydrogenase; HbF: Hemoglobin F; LDH: Lactate Dehydrogenase; AST: Aspartate Aminotransferase; CKD: Chronic Kidney Disease; TRJV: Tricuspid Regurgitant Jet Velocity; ACS: Acute Chest Syndrome; AVN: Avascular Necrosis; VOC: Vaso-Occlusive Crises
Elevated pulse pressure was less frequent in females compared to males in both cohorts (P≤0.0004). History of leg ulcer (P=0.0004) was less frequent in females compared to males in the UIC cohort and TRJV >3m/s was less frequent in females versus males in the Walk-PHaSST cohort (P=0.004). No differences in rates of chronic kidney disease or stroke were observed by sex (Table 1).
Analysis of protein coding autosomal genes in the UIC cohort did not reveal differential expression according to gender at a false discovery rate of 5%. Hemolytic markers did not differ significantly according to G6PD genotype in the UIC cohort, but LDH and AST concentrations differed according to G6PD genotype (G6PD B < G6PD A < G6PD A-) in the Walk-PHaSST cohort (Supplementary Table 2). In neither cohort was there an interaction between G6PD status with sex and the degree of hemolysis (UIC: P=0.8; Walk-PHaSST: P=0.9).
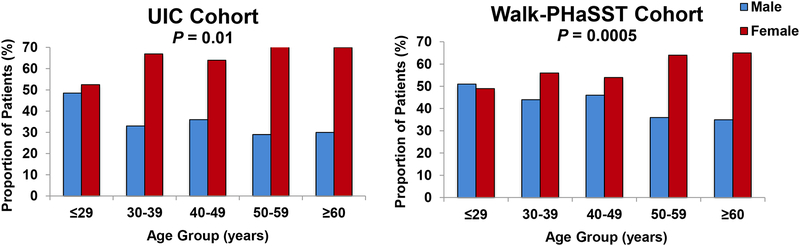
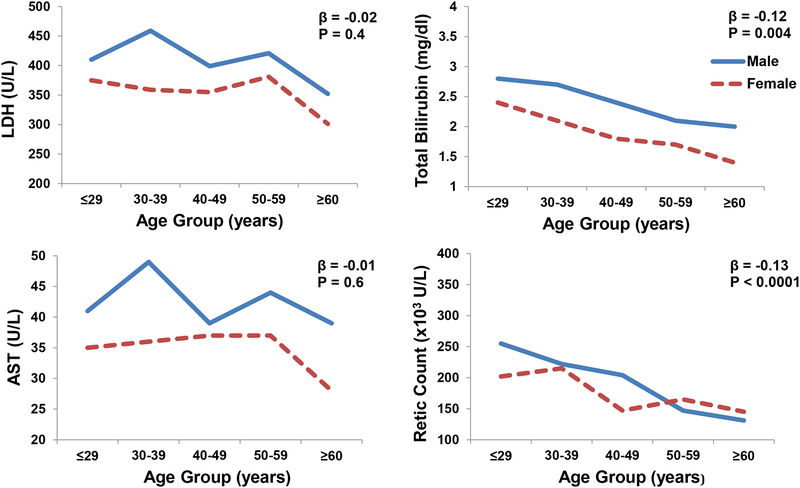
In both the UIC (P=0.01) and Walk-PHaSST (P=0.0005) cohorts, the ratio of females:males progressively increased with increasing age decile (Figure 1A). In both the UIC and Walk-PHaSST cohorts (Figure 1B, 1C), there was a progressive decline in hemolytic markers with incremental age decile; the associations were significant for total bilirubin and absolute reticulocyte counts (P≤0.004).
Figure 1A:
Age of SCD patients categorized by decile in the UIC (A) and Walk-PHaSST (B) cohorts.
Figure 1B:
Hemolytic markers categorized by age decile in the UIC cohort (mean and standard error bars provided).
Figure 1C:
Hemolytic markers by age decile in the Walk-PHaSST cohort (mean and standard error bars provided).
The modest increases in HbF% in females compared to males is consistent with what has been observed in other SCD cohorts and may be partially explained by the H allele on the Xp22.2 F-cell production (FCP) locus.[4] Differences in the degree of hemolysis persisted after adjusting for HbF%. Taken together with no differences in autosomal gene expression or G6PD interaction effects, these findings suggest other sex-specific pathways to explain the difference in hemolytic rate between males and females. Estrogen may provide an anti-oxidant effect protecting RBC from hemolysis. In keeping with this possibility, the anti-estrogen tamoxifen induces hemolysis by disrupting the erythrocyte membrane.[6] There is also evidence that testosterone may play a detrimental effect on RBC stability.[3] Reduced osmotic and oxidative hemolysis was observed in mice after orchiectomy, similar to the degree in female mice, and testosterone replacement restored the hemolytic response. The effects of sex hormones on RBC membrane stability should be analyzed more closely in future studies of SCD patients.
An elevated pulse pressure is a cardiovascular and mortality risk factor in the general population that indicates arterial stiffness. Our finding of lower pulse pressures in females compared to males in both cohorts is consistent with our observation of a lower prevalence of TRJV >3.0 m/s in females from the Walk-PHaSST cohort, but we did not observe an association of sex with stroke or CKD in either cohort.
Markers of hemolysis decreased and the ratio of females:males increased with increasing age decile in both cohorts. The ratio of females:males in SCD patients >50 years-old trended higher in the UIC (ratio=2.3, P=0.08) and Walk-PHaSST cohorts (ratio=1.8, P=0.05) compared to the general African American population (ratio=1.2, 2010 U.S. Census Bureau).
Limitations to this study are that the analyses were cross-sectional in nature and the clinical information was gathered from patients’ medical history. Our results suggest that future investigation of loci on the sex chromosomes for effects on HbF% and the degree of hemolysis is warranted. Understanding the differences in the sex chromosomes and their effects on RBC stability and hemolysis may provide insight into novel therapies for SCD.
Supplementary Material
Acknowledgements:
The project described was supported by the National Institutes of Health through grants K23HL125984 (S.L.S), R01HL111656 and R01HL127342 (R.F.M.). The Walk-PHaSST project was supported by federal funds from the National Heart, Lung, and Blood Institute under contract HHSN268200617182C. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References:
- 1.Arias E, Heron M, Xu J. United States Life Tables, 2013. Natl Vital Stat Rep 2017;66:1–64. [PubMed] [Google Scholar]
- 2.Gladwin MT, Barst RJ, Gibbs JS, et al. Risk factors for death in 632 patients with sickle cell disease in the United States and United Kingdom. PLoS One 2014;9:e99489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanias T, Sinchar D, Osei-Hwedieh D, et al. Testosterone-dependent sex differences in red blood cell hemolysis in storage, stress, and disease. Transfusion 2016;56:2571–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dover GJ, Smith KD, Chang YC, et al. Fetal hemoglobin levels in sickle cell disease and normal individuals are partially controlled by an X-linked gene located at Xp22.2. Blood 1992;80:816–824. [PubMed] [Google Scholar]
- 5.Motulsky AG, Yoshida A, Stamatoyannopoulos G. Variants of glucose-6-phosphate dehydrogenase. Ann N Y Acad Sci 1971;179:636–643. [DOI] [PubMed] [Google Scholar]
- 6.Cruz Silva MM, Madeira VM, Almeida LM, et al. Hemolysis of human erythrocytes induced by tamoxifen is related to disruption of membrane structure. Biochim Biophys Acta 2000;1464:49–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.