Figure 3. A cellular model of insulin signaling via TRPC5 channel activation in POMC neurons.
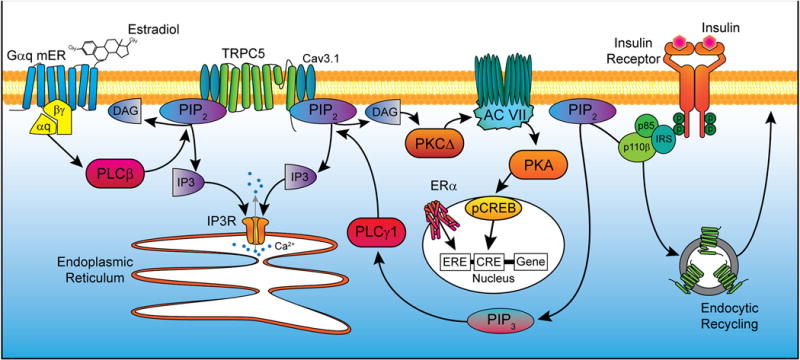
Activation of a Gq-mER by E2 stimulates phospholipase C β (PLCβ) to catalyze the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into diacylglycerol and inositol 1,4,5-trisphosphate (IP3). IP3 activates the IP3 receptor, causing release of calcium (Ca2+) from the endoplasmic reticulum. DAG drives protein kinase CΔ to increase the activity of cAMP production by adenylyl cyclase VII which in turn drives protein kinase A activity (PKA). cAMP response element-binding protein (CREB) in the nucleus is phosphorylated, allowing for interactions with certain DNA sequences to alter gene transcription. Alternatively, ERα can dimerize after binding E2 and act on estrogen response elements (ERE) in the nucleus to similarly affect expression of target genes. Insulin signals via insulin receptor substrate-phosphoinositide 3 kinase (IRS-PI3K) to activate TRPC5 channels in POMC neurons, which generates a robust inward cationic current to depolarize POMC neurons and increase their excitability. PI3K (p85/p110) will also accelerate the rapid insertion of TPRC5 channels into the plasma membrane (Bezzerides et al., 2004). E2 facilitates TRPC channel activity through upregulation expression of Cav3.1 (T-type calcium) channels and PLC catabolism of PIP2 to facilitate TRPC5 channel opening via ERα and Gαq-mER, respectively.
