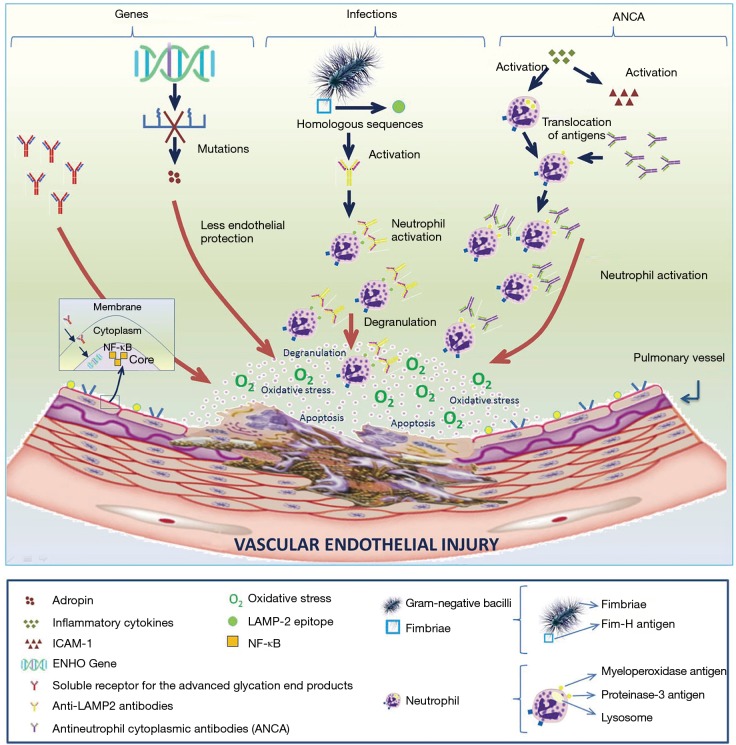
Figure 1.
Etiopathogenesis of pulmonary vasculitis. Genetic, infectious and immune factors are involved in the etiopathogenesis of vasculitis, with a major role of antineutrophil cytoplasmic antibodies (ANCAs). (I) Genetic factors: genetic mutations in the energy homeostasis associated gene reduces the release of adropine, thereby reducing endothelial protection and promoting vascular damage. In the same way, raises the levels of soluble RAGE, which promotes the production of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-ĸß) and initiates the inflammatory cascade involved in vascular damage; (II) infectious factors: Gram-negative bacilli fimbriae have FimH antigens that have DNA homologous sequences with epitopes (antigenic determinants that are recognised by the immune system) of the lysosomal-associated membrane protein 2 (LAMP-2). In light of an infection by these germs, FimH antigens are falsely recognised as LAMP-2 epitopes, which will activate the production of anti-LAMP-2 antibodies. This situation will trigger neutrophil activation and subsequent degranulation, finally producing vascular cellular apoptosis; (III) immunologic factors: vasculitides are associated with an acute proinflammatory status, with elevated levels of cytokines (ICAM-1, VCAM-1, IL-1, etc.) that take part in neutrophil activation, thereby promoting antigenic translocation (proteinase-3 antigen and myeloperoxidase pass from the lysosome to the cell membrane). In this new location, antigens are accessible to ANCAs. This phenomenon results in neutrophil degranulation and oxidative stress, causing cellular apoptosis and endothelial vascular damage. RAGE, receptor of advanced glycated end products.