Fig. 1.
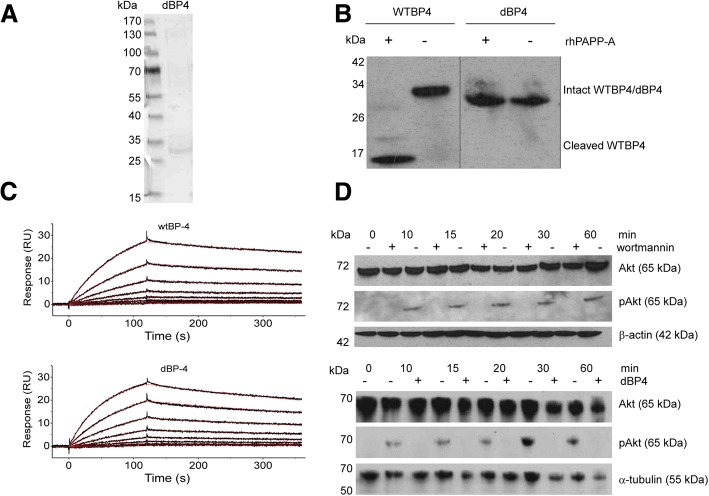
dBP4 binds IGF1, is resistant to PAPP-A cleavage and inhibits IGF-induced Akt phosphorylation. a SDS-PAGE analysis of purified dBP4 protein. b dBP4 or wtIGFBP4 (WTBP4) were treated with recombinant PAPP-A in the presence of IGF1 for 24 h. Intact IGFBP4 and cleavage fragments were identified by western blot with anti-IGFBP4 antibody. Position of molecular weight (kDa) markers is indicated. Empty lanes on the blot were removed between WTBP4 and dBP4 for clarity. c Surface plasmon resonance. Twofold serial dilutions of purified HIS-tagged mouse wtIGFBP4 or dBP4 (50 nM to 0.4 nM) were injected over a surface with immobilized mouse IGF1. Recorded binding curves are shown in black and a global 1:1 L fit is shown in red. d 25 μg of cell lysate was fractionated by SDS-PAGE, and total Akt or pAkt identified by western blot. (upper panel) 4T1.2luc cells were pre-treated with the PI3K inhibitor, wortmannin, followed by IGF1 (100 ng/ml). β-actin is shown as loading control. (lower panel) 4T1.2luc cells were treated with IGF1 (100 ng/ml) or IGF1 pre-incubated with 2.5 μg/ml dBP4. α-tubulin is shown as loading control. Blots are representative of three independent experiments