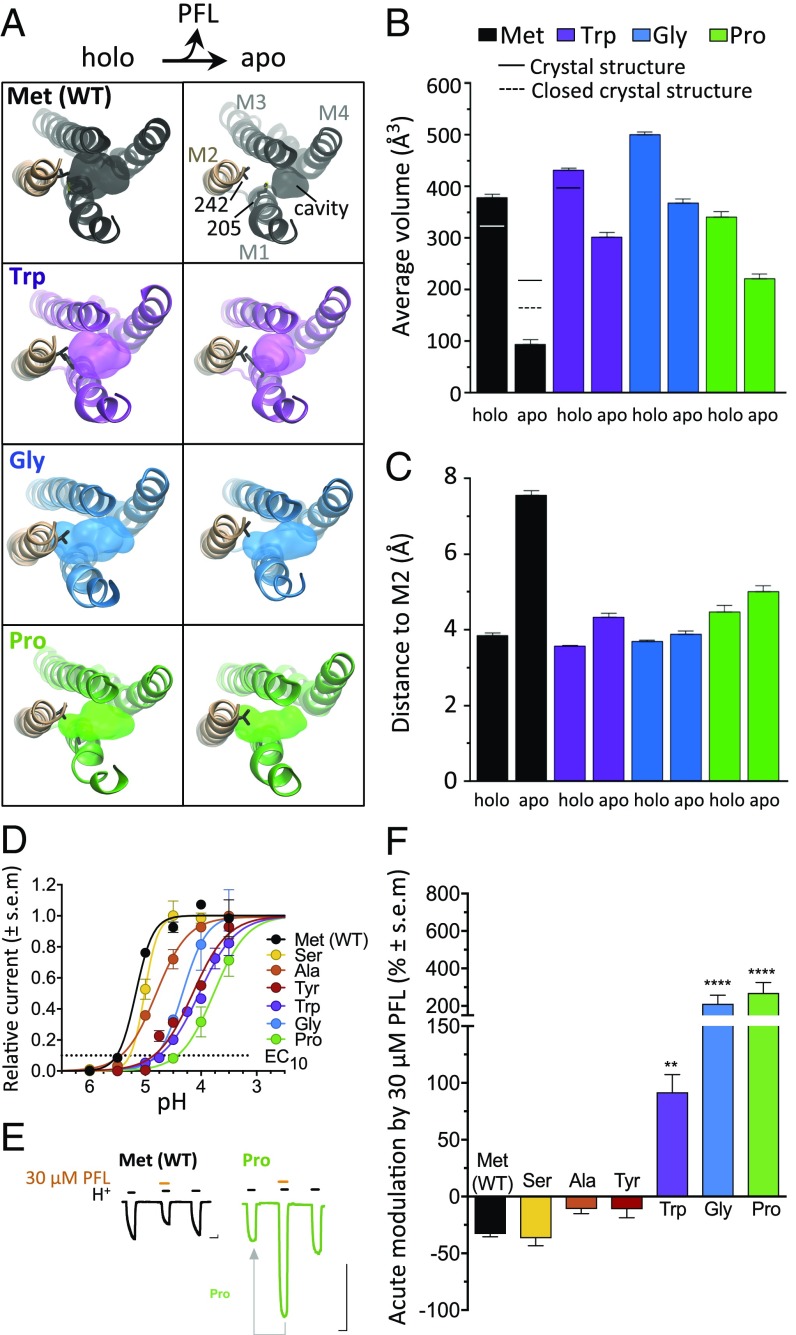
Fig. 2.
M1-205 mutations increase membrane access to M2 and enable PFL potentiation. (A) Top views as in Fig. 1B of representative frames from holo (Left) and apo (Right) simulations for Met (WT) and M1-205 variants Trp, Gly, and Pro. Surfaces show the PFL binding cavity, with residues M1-205 and M2-242 as sticks. (B) Mean intrasubunit cavity volumes for simulations (columns) and previous X-ray structures (lines). (C) Mean shortest distance between the pocket perimeter and M2 Val-242. In B and C, columns represent mean values ± block error estimates. (D) Normalized concentration−response curves for H+ activation of M1-205 variants (EC10 as dotted line). (E) Sample traces for WT (black) and Pro-205 (green) showing EC10 activation pretreatment, then cotreatment with 30 μM PFL, then posttreatment. Arrow indicates comparison in F. (Scale bars, 1 μA vs. 2 min.) (F) Acute modulation of GLIC M1-205 variants by 30 μM PFL, calculated as percent change in cotreatment vs. pretreatment currents. Significance is relative to WT, one-way analysis of variance, n = 4 to 8 (**P < 0.01; ****P < 0.0001).