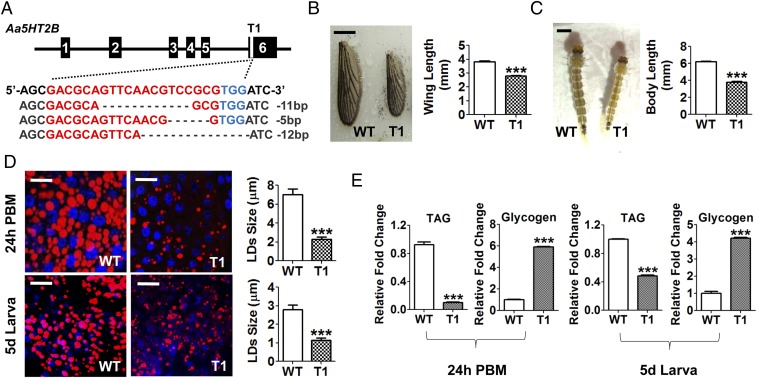
Fig. 2.
Genomic disruption of Aa5HT2B by CRISPR-Cas9 resulted in a reduced body size and diminished lipid storage. (A) Sequence alignment of a sgRNA-targeted (named T1) genomic region. Exons are shown in black boxes. The sequence of the sgRNA target site (labeled in red) is in the last exon, where the receptor C terminus is located to transmit serotonin signals. The protospacer adjacent motif (PAM) sequence is in blue. (B) Comparison of wing length of WT control and ΔAa5HT2B-T1 mutant adults. (Scale bar, 1 mm.) (C) ΔAa5HT2B-T1 larvae also exhibit reduced body length. (Scale bar, 1 mm.) Shown are the last-instar larvae on the fifth day after egg hatching (wandering fourth-instar larval stage). All surviving individuals were used for measurements. (D) Lipid droplets in the fat-bodies dissected from WT and T1 mutant adults and larvae (with reduced body size) at 24-h PBM and the fifth day after egg hatching were detected by Nile red staining and visualized under a Leica SP5 confocal microscope. (Scale bars, 25 μm.) Blue, nuclear staining with DAPI. The lipid droplet sizes (LDs) are significantly lower in T1 mutant females than in WT. (E) TAG levels are significantly less and the glycogen levels significantly higher in T1 mutant females than in WT. Data represent three biological replicates (six individuals in each replication for LDs, TAG, and glycogen measurement) with three technical replicates and are shown as mean ± SEM. ***P < 0.001.