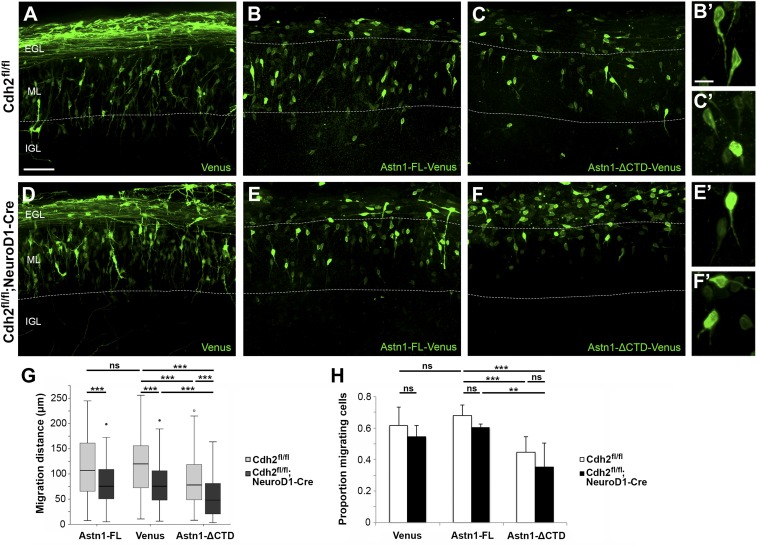
Fig. 5.
ASTN1 and CDH2 interact functionally to regulate migration. Organotypic slice cultures from the cerebellum of P8 Cdh2fl/fl and Cdh2fl/fl;NeuroD1-Cre mice were electroporated with Venus (A and D), Astn1-FL-Venus (B and E), or Astn1-ΔCTD-Venus (C and F). ASTN1-Venus fluorescence labeled the cell soma and processes but not the parallel fibers. After 60 h, the distance migrated by GCPs expressing Astn1-FL-Venus was similar to that of GCPs expressing Venus (A, B, and G) both in the presence and absence of neuronal Cdh2 (D, E, and G). However, in slice cultures of control mice where GCPs expressed Astn1-ΔCTD-Venus, GCPs migrated a 35% shorter distance (78 µm median) than GCPs expressing Venus (120 µm median) (C and G). Loss of Cdh2 combined with overexpression of Astn1-ΔCTD-Venus in GCPs resulted in more severe migration defects, indicated by a 60% reduction in the median migration distance (48 µm) and a higher number of cells stalled in the EGL (F and G). (H) A significantly higher proportion of cells expressing Astn1-ΔCTD-Venus were rounded or multipolar. Dotted lines indicate EGL/ML and ML/IGL boundaries. **P < 0.01; ***P < 0.001; ns, not significant. (Scale bars: 50 µm in A–F; 10 µm in B′, C′, E′, and F′.)