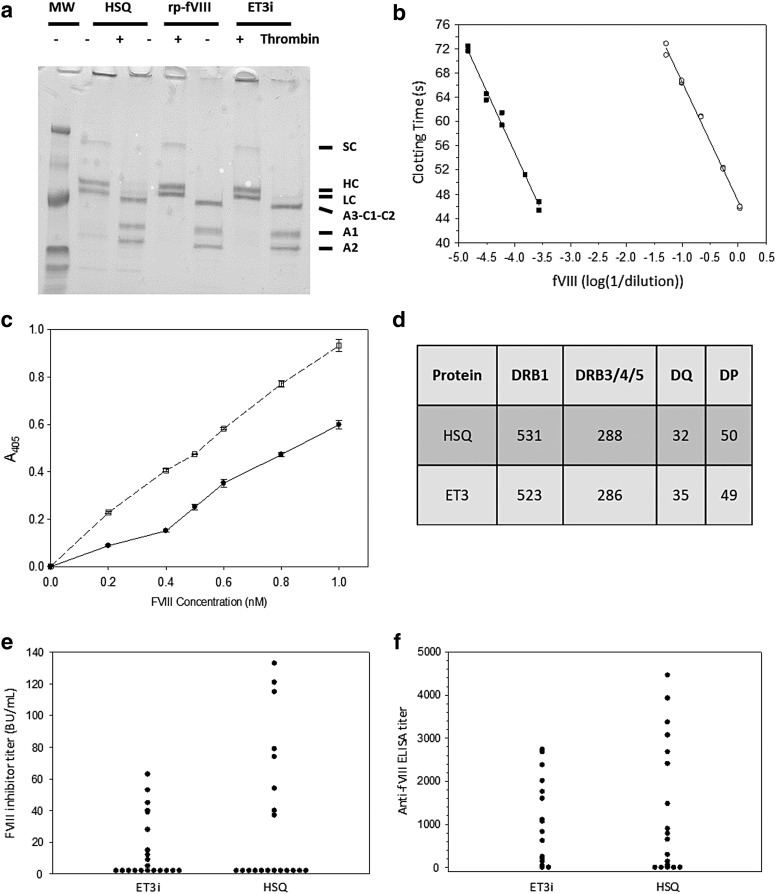
Figure 1.
Recombinant ET3 (ET3i) biochemistry and immunogenicity studies. (a) Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis of recombinant human BDD fVIII (HSQ), B-domain-deleted (BDD) recombinant porcine (rp) factor VIII (fVIII), and ET3i—2 μg HSQ, rp-fVIII,18 and ET3i were subjected to 4–15% gradient SDS-PAGE under reducing conditions and visualized by Coomassie Blue staining. “+ Thrombin” indicates samples that were treated with 100 nM porcine thrombin for 5 min before analysis. Single chain (SC), heavy chain (HC), light chain (LC), A3-C1-C2, A1, and A2 polypeptides are labeled. The lane designated “MW” contains molecular weight markers of 207, 129, 75, 39.7, 32.1, and 17.5 kDa. (b) fVIII activity assay of ET3i. The clotting times of dilutions of 0.76 μM ET3i (closed squares) into fVIII-deficient plasma were measured and compared to a standard curve made by dilutions of pooled normal human plasma (open circles). The lines represent linear regression fits to the data. (c) Binding of ET3i and HSQ to human von Willibrand factor (vWf). ET3i (open squares) or HSQ (closed circles) were added to human vWF immobilized on microtiter wells, followed by detection of bound fVIII using anti-fVIII monoclonal antibodies as described in the Methods. (d) HSQ and ET3 amino acid sequences were analyzed for predicted T-cell epitopes. The number of critical fVIII epitopes predicted for each human leukocyte antigen gene family are shown. (e and f) Hemophilia A mice received intravenous injections of 1 μg ET3i or HSQ every 4 weeks. One week following the final dose, plasma samples were collected and assayed for anti-fVIII inhibitor titers (e) and anti-fVIII immunoglobulin G titers (f). No significant differences were observed: p = 0.8 and 0.9, respectively, in (e) and (f).