Abstract
Rare sugars are defined as monosaccharides and their derivatives, which rarely exist in nature and have various beneficial effects on organisms, biomaterials and foods. Glycolipids are composed of sugars and lipids and have been intensively studied in various fields such as environmental engineering, nanotechnology and molecular biology. Here, we synthesise new types of glycolipids composed of rare sugars, glycerol and lipids (RSGLs), using 6 different types of rare sugars by combining the modified Fischer and lipase reverse reactions. We confirm the production of RSGLs by thin layer chromatography (TLC), Fourier-transform infrared (FT-IR) spectroscopy and matrix assisted laser desorption/ionisation time of flight mass spectroscopy (MALDI-TOF-MS) and investigate the cytotoxicity of RSGLs by lactate dehydrogenase (LDH) and alamar blue assays. We successfully synthesise novel RSGLs; i.e., D-ribose-glycorol-lipid, D-allose-glycorol-lipid, L-rhamnose-glycorol-lipid, L-lyxorse-glycorol-lipid, L-gulose-glycorol-lipid and L-fucose-glycorol-lipid. We finally clarify the effect of the concentration of those RSGLs on cytotoxicity, which is of great importance considering the utilisation of RSGLs particularly in the field of biomedicine.
Keywords: Organic chemistry, Natural product chemistry, Materials chemistry
1. Introduction
Rare sugars, which are defined as monosaccharides and their derivatives by the International Society of Rare Sugars (ISRS), rarely exist in nature. Several efficient methods of producing rare sugars have been invented and established since D-tagatose-3-epimerase was discovered [1, 2]. Such enzymatic mass production of rare sugars has reduced dramatically the cost of products, thanks to which the activity of research related to rare sugars has been remarkably promoted in the world. Rare sugars have now become one of the most common research subjects in biotechnology, and food and medical science since it has been revealed that rare sugars possess various beneficial effects on medical treatment and health control such as the suppression of hyperglycemia [3], the suppression of proliferation of cancer cells [4] and the protection of teeth [5]. D-tagatose and D-psicose have been accepted as “Generally Recognised As Safe (GRAS)” products by the Food and Drug Administration (FDA) and therefore they have been actively used in various types of foods.
Rare sugars can be used for the synthesis of functional materials. For example, D-psicose has been used for the synthesis of novel disaccharides; e.g., D-psicose disaccharide was previously synthesised by chemical reaction [6], whereas D-xylose-D-psicose and D-glucose-D-psicose disaccharides were, respectively, synthesised using endo-1,4-β-D-xylanase [7] and cyclomaltodextrin glucanotransferase [8].
Several chemical methods of synthesising glycolipids have been developed [9, 10, 11] and glycolipids have already been used in various research fields such as environmental engineering [12], nanotechnology [13] and molecular biology [14]. Glycolipids are now expected to be utilised in biomedical fields. However, the cytotoxicity of glycolipids has not yet been investigated although the chemical characteristics and structures of synthesised glycolipids have been analysed in detail. In this study, we synthesise new types of glycolipids composed of rare sugars, glycerol and lipids (RSGLs) and investigate the chemical structures and cytotoxicity of RSGLs aiming to utilise them in the fields of biotechnology and medical science and engineering in the near future.
2. Materials and methods
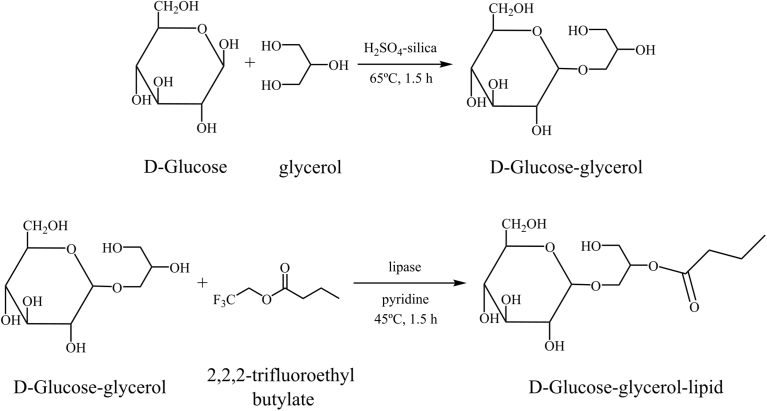
We synthesised RSGLs following the procedure described by Roy et al. [15] and Colombo et al. [9]. The synthetic procedure is shown in Fig. 1. We conjugated sugar and glycerol by the modified Fischer method using D-glucose (Kanto Chemical Co.) and 6 types of rare sugars; i.e., D-ribose (Wako Pure Chemical Industries, Ltd.), D-allose (Tokyo Chemical Industry Co., Ltd.), L-rhamnose (Nacalai tesque), L-lyxorse (Tokyo Chemical Industry Co., Ltd.), D-gulose (Tokyo Chemical Industry Co., Ltd.), and L-fucose (Nacalai Tesque, Inc.).
Fig. 1.
Synthetic procedure of a glycolipid composed of D-glucose, glycerol and lipid.
0.5 mol of glycerol (Wako Pure Chemical Industries, Ltd.), 2.22 mmol of each of the above sugars and 5 mg of H2SO4-silica, which had been prepared by mixing 50 mL of diethyl ether (Wako Pure Chemical Industries, Ltd.), 3 mL of sulphuric acid (Wako Pure Chemical Industries, Ltd.) and 10 g of Merck 60 silica gel (0.040–0.063 mm), were mixed and heated at 65 °C for 1.5 h with a constant stirring and then the solvent was evaporated in vacuum. 400 μL of the reactants were charged on the silica gel and washed twice using 1 mL of dichloromethane (Sigma-Aldrich Co. LLC.) and 1 mL of a mixture of dichloromethane-methanol (8:1). Then, sugar-glycerol conjugates were extracted with 2 mL of a mixture of dichloromethane-methanol (8:3). We confirmed the production of each rare sugar-glycerol conjugate by thin layer chromatography (TLC) (Merck 60 F254 plates 0.25 mm thickness), having placed each product on a TLC plate and developed it with n-BuOH-EtOH-water (6:6:1). We then synthesised glycolipids composed of rare sugars, glycerol and lipids as follows; (1) 0.39 mmol of the rare sugar-glycerol conjugate, 1.17 mmol of 2,2,2-trifluoroethylbutyrate (Wako Pure Chemical Industries, Ltd.) and 475 U of lipase immobilised on a macroporous acrylic resin (Sigma-Aldrich Co. LLC.) were mixed with 2 mL of pyridine (Wako Pure Chemical Industries, Ltd.); (2) the mixture was incubated at 45 °C for 1.5 h; and (3) the reaction products were concentrated with a centrifugal evaporator and purified by silica gel column chromatography using methanol.
We confirmed the production of each RSGL by TLC using a mixture of dichloromethane-methanol (8:2) as an eluent. We then characterised the chemical structure of each RSGL by Fourier transform infrared spectroscopy (FT-IR) (Thermo iS50 ABX, Thermo Fisher Scientific, Inc.) and matrix assisted laser desorption/ionisation time of flight mass spectroscopy (MALDI-TOF-MS) (autoflex speed TOF/TOF, Bruker, Co.). The mass spectrum of each RSGL was measured on the linear mode, whereas the tandem mass spectrum was measured on the reflection mode. Note that 2,5-dihydroxy benzoic acid was used as a matrix. We also investigated the cytotoxicity of each RSGL by a lactose dehydrogenase (LDH) assay (Cytotoxicity LDH Assay Kit-WST, Dojindo Molecular Technologies, Inc.) and alamar blue assay (Bio-Rad Laboratories, Inc.). We cultivated IM-9 human myeloma multiple cells in an RPMI-1640 medium (Sigma-Aldrich Co. LLC.) supplemented with 10% fatal bovine serum (Biowest), 1% streptomycin/penicillin (Life Technologies Corporation), 1% GulutaMax (Life Technologies Corporation) and 1% sodium pyruvate (Life Technologies Corporation) at 37 °C for 2 days in a CO2 incubator. The IM-9 cell suspension and 1, 5, 10, 15, 20, 25 and 30 mM of each RSGL having been introduced into 96 wells, a mixture of the cell suspension and RSGLs were incubated at 37 °C for 5 h in a CO2 incubator. After incubation, we estimated the activity of LDH released from the cells by measuring absorbance at 490 nm wavelength by a plate reader (Spectra Max i3x, Molecular Devices, LLC.). We also analysed the effect of RSGLs on the metabolism of IM-9 cells by an alamar blue assay. A mixture of the cell suspension, the concentration of which was 5 × 105 cells/mL, and 1, 5, 10, 15, 20, 25 and 30 mM of each RSGL was incubated at 37 °C for 5 h in a CO2 incubator and then 10 μL of alamar blue was added to the mixture. After incubation of the mixture at 37 °C for 3 h in a CO2 incubator, we estimated the cell metabolic activity by measuring the fluorescence of the alamar blue reagent at 560 nm excitation and 590 nm emission wavelengths by a plate reader.
The surface tension of the media was measured by a contact angle measurement system (DM-701, Kyowa Interface Science Co., LTD.) to investigate the dependence of the surface tension of the media on the concentration of RSGLs and to clarify the relation between the cytotoxicity of RSGLs and the surface tension of the media.
3. Result and discussion
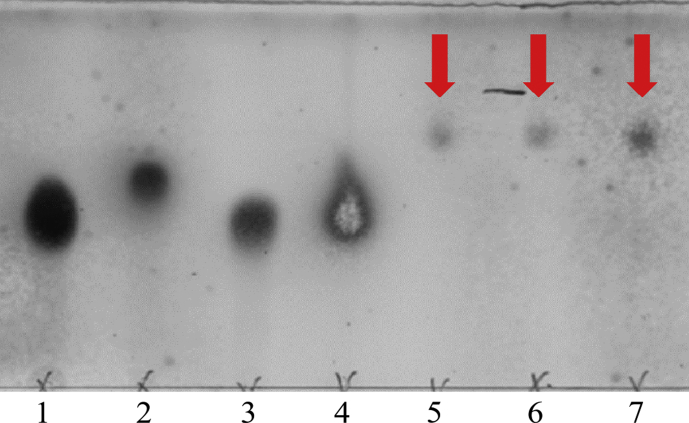
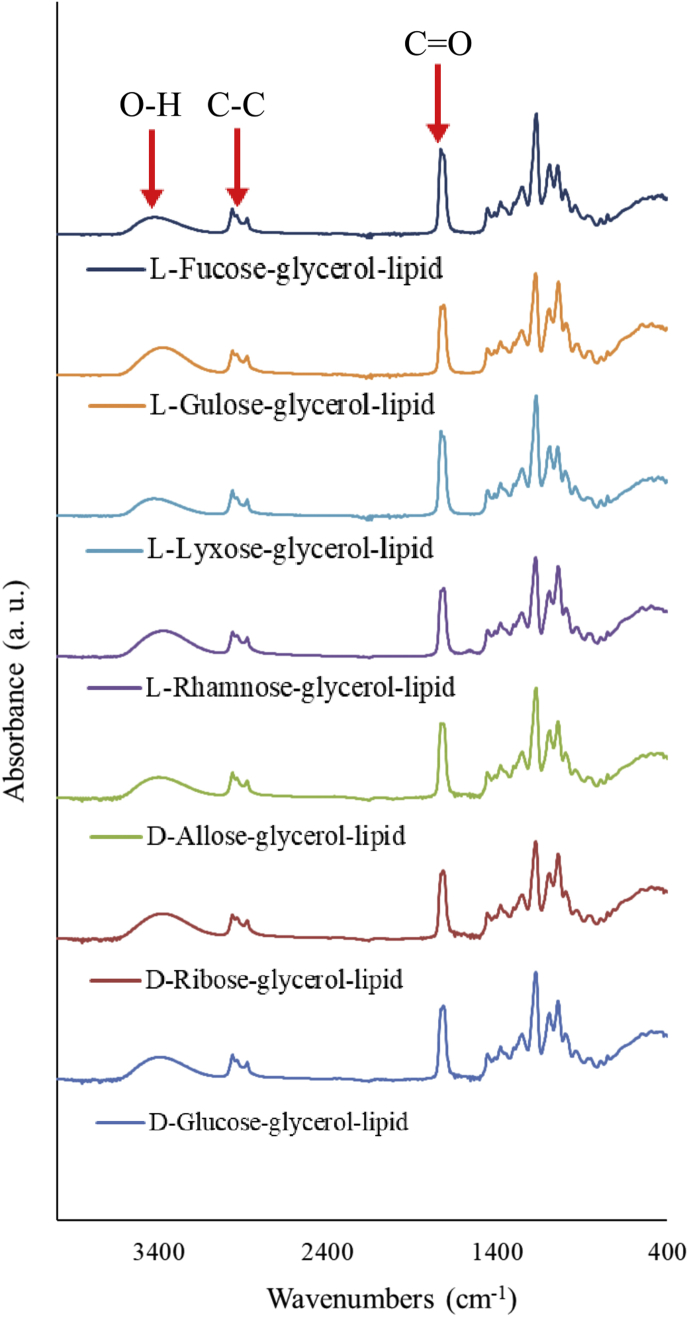
First of all, we confirmed by TLC that rare sugar-glycerol conjugates were produced (see Fig. 2, where the spots indicated by arrows correspond to D-glucose-glycerol, D-ribose-glycerol and D-allose-glycerol). The FT-IR spectra of the freeze dried RSGLs are shown in Fig. 3. The absorption band at around 3400 cm−1 corresponds to the O-H stretching, which is derived from rare sugars and glycerol in RSGLs, whereas another one at 1800 cm−1 represents the ester C=O stretching from butyrate in RSGLs. Note that there is O-H bonds, but no C=O bond in sugars and glycerol, while 2,2,2-trifluoroethylbutyrate has C=O residue, but no O-H bond.
Fig. 2.
Glycoglycerol detected by thin layer chromatography. 1; D-glucose, 2; D-ribose, 3; D-allose, 4; glycerol. The spots indicated by arrows represent sugar-glycerol conjugates; that is, 5; D-glucose-glycerol, 6; D-ribose-glycerol, and 7; D-allose-glycerol.
Fig. 3.
FT-IR spectra of freeze dried glycolipids. The spectra of RSGLs were analysed with diamond Attenuated Total Reflection.
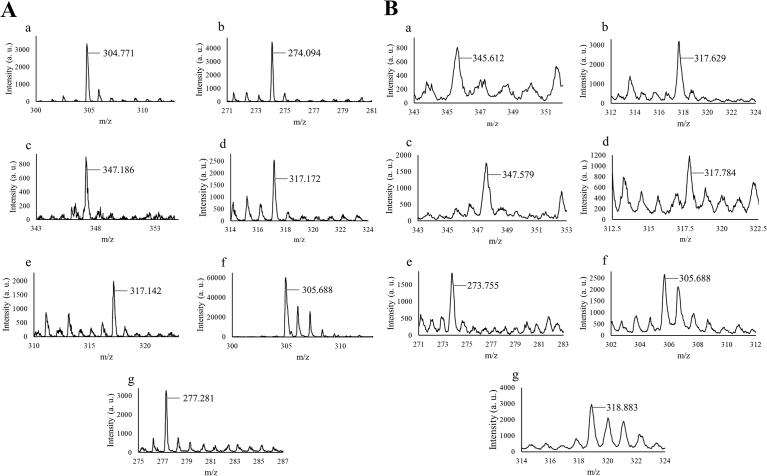
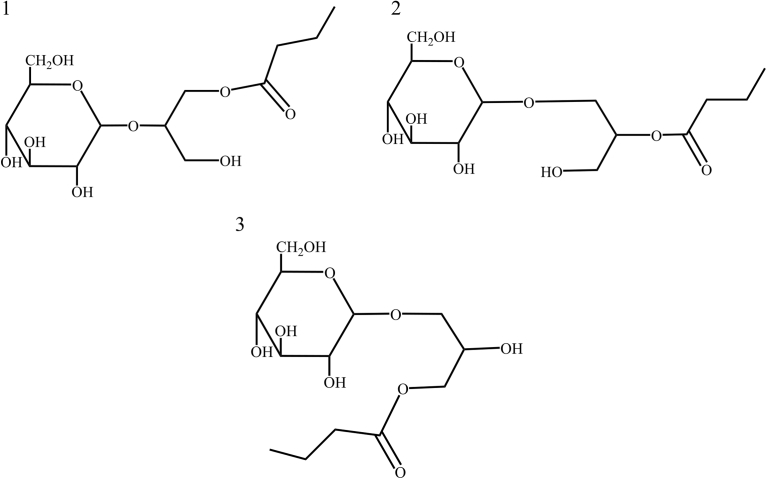
The tandem mass spectra of RSGLs obtained by the reflection mode of MALDI-TOF-MS are shown in Fig. 4A, whereas the mass spectra of RSGLs measured by the linear mode is also presented in Fig. 4B. The mass spectra of the RSGLs presently produced agreed with those of theoretically predicted ones (Compass IsotopePattern 2.0, Bruker, Co.). Thus, RSGLs were successfully synthesised for the first time according to the spectra obtained by both FT-IR and MALDI-TOF-MS. Previous reports revealed that there are several isomers in chemically synthesised glycolipids [9, 10, 16], and therefore it is supposed that there are also several isomers among the present RSGLs (see Fig. 5). The structures of the RSGLs will be identified in more detail using more purified samples by nuclear magnetic resonance (NMR) spectroscopy.
Fig. 4.
Mass spectra of rare sugar-glycerol-lipids measured by MALDI-TOF-MS. A. Tandem mass spectra measured on the reflection mode. B. Mass spectra measured on the linear mode. a; D-glucose-glycorol-lipid, b; D-ribose-glycorol-lipid, c; D-allose-glycorol-lipid, d; L-rhamnose-glycorol-lipid, e; L-lyxorse-glycorol-lipid, f; L-gulose-glycorol-lipid, g; L-fucose-glycorol-lipid.
Fig. 5.
Isomers of a glycolipid composed of D-glucose, glycerol and lipid.
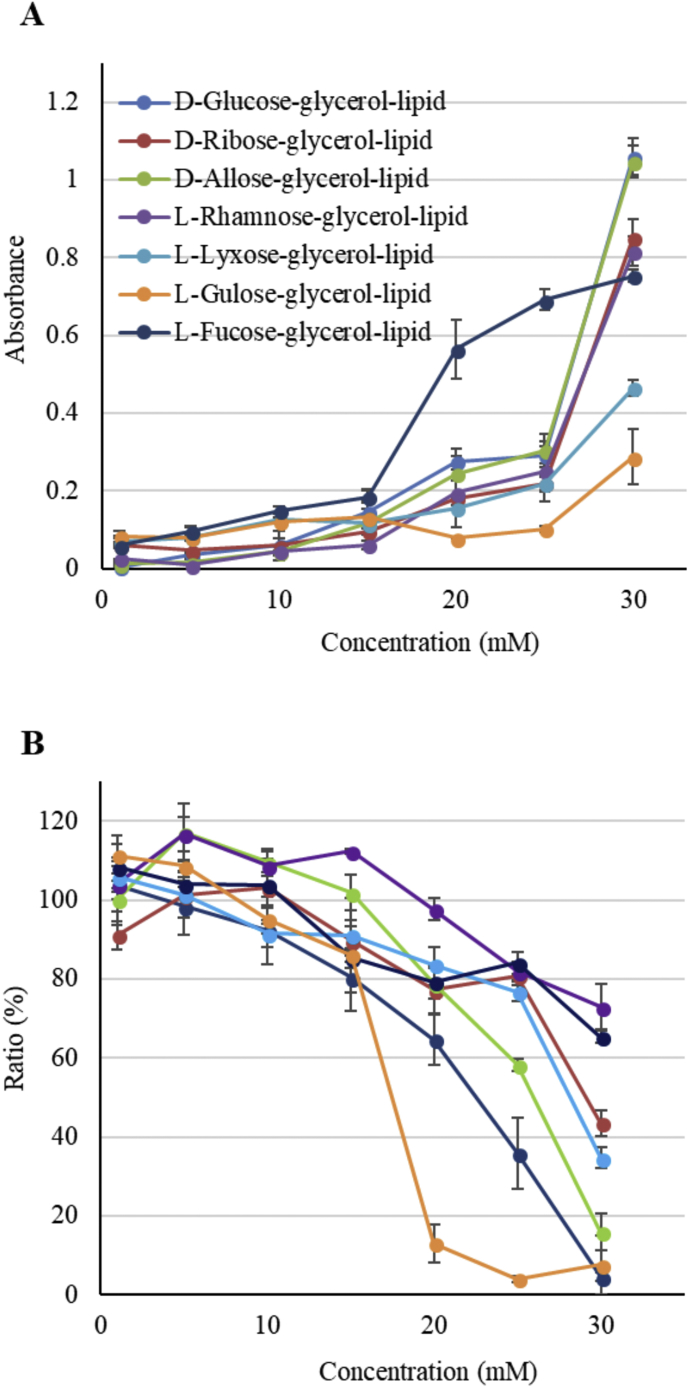
We investigated the cytotoxicity of RSGLs using IM-9 human myeloma multiple cells by LDH and alamar blue assays as mentioned. The LDH activity and cell metabolic activity are, respectively, shown in Fig. 6. The cytotoxicity of RSGLs was low when the concentration of RSGLs was lower than 15 mM, whereas it increased as the concentration exceeded 20 mM except for L-fucose-lipid, in which case the cytotoxicity increased rapidly when the concentration was over 15 mM.
Fig. 6.
Cytotoxicity of rare sugar-glycerol-lipids compounds against IM-9 cells. A; Lactate dehydrogenase assay. The ordinate represents the level of damage to the cell membranes. B; Alamar blue assay. The ordinate represents the metabolic activity of cells.
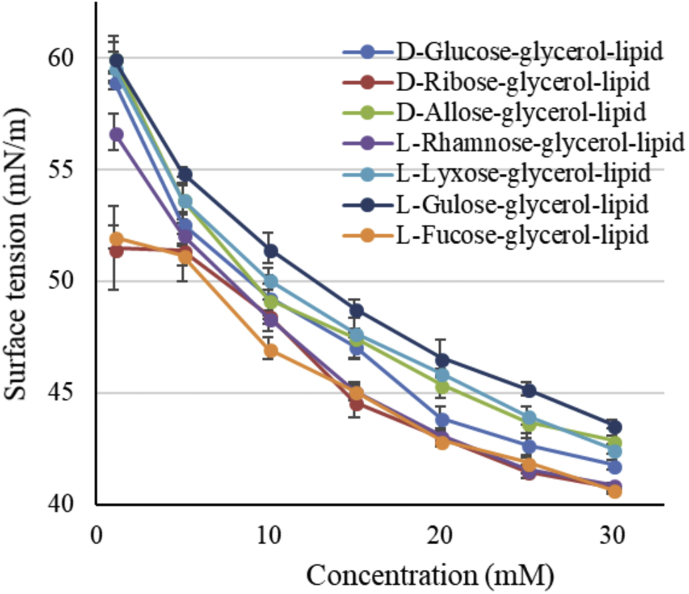
It is well known that D-allose inhibits the growth of cancer cells by overnight treatment when the concentration is greater than 50 mM [4]. It was shown in the present study that the cytotoxicity of L-fucose-lipid was higher than that of D-allose lipid when the concentration of L-fucose lipid ranged from 15 to 25 mM. It is known that neither sugars, excluding D-allose, nor butyrates have inhibitive effect on the cancer growth, while in the present study RSGLs showed toxicity against IM-9 cells. Jiang et al. demonstrated that the toxicity of rhamnolipids increased when the surface tension of the cell culture medium was lower than 41 mN/m [17]. The dependence of the surface tension of the media on the concentration of RSGLs produced in the present study is shown in Fig. 7. It is clearly shown that the surface tension of the media decreased with an increase in the concentration of RSGLs, noting that the cytotoxicity of RSGLs also increased as the concentration of RSGLs increased, which indicates that the cytotoxicity of RSGLs increases with a decrease in the surface tension of the media. However, the surface tension of the present media was higher than 41 mN/m even when the concentration of the RSGLs was 30 mM. What is more, the level of cytotoxicity of RSGLs was not directly related to the difference in the value of the surface tension of the media. It is therefore supposed that the secondary structures such as micelles formed by RSGLs in the media as well as the reduction of the surface tension of the media may be responsible for the cause of the toxicity of RSGLs against the cells, considering the amphiphilic structures of the present RSGLs [18].
Fig. 7.
Dependence of the surface tension of the media on the concentration of rare sugar-glycerol-lipids dispersed in the media.
In summary, we successfully synthesised new types of glycolipids and investigated the cytotoxicity of those glycolipids. We will be characterising and analysing the structures of the RSGLs in more detail by NMR spectroscopy. The mechanism of the cytotoxic behaviour of the glycolipids is still an open question. We will also be investigating the mechanism in detail so that the presently produced glycolipids may be utilised in biomedical fields; e.g., the present glycolipids may be used as nano vehicles for cancer treatment.
Declarations
Author contribution statement
Keisuke Hirata: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Takashi Uchida, Yoshikata Nakajima: Analyzed and interpreted the data.
Toru Maekawa: Analyzed and interpreted the data; Wrote the paper.
Toru Mizuki: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by a grant for the Program for the Strategic Research Foundation at Private Universities S1101017 organised by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, and the Inoue Enryo Memorial Grant, Toyo University, Japan.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Itoh H., Okaya H., Khan A.R., Tajima S., Hayakawa S., Izumori K. Purification and characterization of D-tagatose 3-epimerase from Pseudomonas sp. ST-24. Biosci. Biotech. Biochem. 1994;58:2168–2171. [Google Scholar]
- 2.Izumori K. Bioproduction strategies for rare hexose sugars. Naturwissenschafte. 2002;89:120–124. doi: 10.1007/s00114-002-0297-z. [DOI] [PubMed] [Google Scholar]
- 3.Matsuo T., Izumori K. Effects of dietary D-psicose on diurnal variation in plasma glucose and insulin concentrations of rats. Biosci. Biotech. Biochem. 2006;70:2081–2085. doi: 10.1271/bbb.60036. [DOI] [PubMed] [Google Scholar]
- 4.Sui L., DONG Y., Watanabe Y., Yamaguchi F., Hatano N., Izumori K., Tokuda M. Growth inhibitory effect of D-allose on human ovarian carcinoma cells in vitro. Anticancer Res. 2005;25:2639–2644. [PubMed] [Google Scholar]
- 5.Tange T., Sakurai Y., Hirose M., Noro D., Igarashi S. The effect of xylitol and fluoride on remineralization for primary tooth enamel caries in vitro. Pediatr. Dent. J. 2004;14:55–59. [Google Scholar]
- 6.Ueda A., Yamashita T., Uenishi J. Chemical synthesis of β-D-psicofuranosyl disaccharides. Carbohydr. Res. 2010;345:1722–1729. doi: 10.1016/j.carres.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 7.Oshima H., Kimura I., Izumori K. Synthesis and structure analysis of novel disaccharides containing D-psicose produced by endo-1,4-β-D-xylanase from Aspergillus sojie. J. Biosci. Bioeng. 2006;101:280–283. doi: 10.1263/jbb.101.280. [DOI] [PubMed] [Google Scholar]
- 8.Oshima H., Kimura I., Morimoto K., Izumori K. Synthesis and structure analysis of glucosylpsicose produced by cyclomaltodextrin glucanotransferase. J. Appl. Glycosci. 2008;55:1–3. [Google Scholar]
- 9.Colombo D., Ronchetti F., Antonio S., Ida T., Lucjo T. A facile lipase catalyzed access to fatty acid monoesters of 2-O-β-D-glucosylglycerol. Tetrahedron Asymmetry. 1996;7:771–777. [Google Scholar]
- 10.Degn P., Pedersen P.H., Duus J.O., Zimmermann W. Lipase-catalysed synthesis of glucose fatty acid esters in tert-butanol. Biotech. Letters. 1999;21:275–280. [Google Scholar]
- 11.Mangunuru P.R.H., Yerabolu R.J., Liu D., Wang Guijun. Synthesis of a series of glucosyl triazole derivatives and their self-assembling properties. Tetrahedron Lett. 2015;56:82–85. [Google Scholar]
- 12.Haryanto B., Chang C.H. Removing adsorbed heavy metal ions from sand surfaces via applying interfacial properties of rhamnolipid. J. Oleo Sci. 2015;64:161–168. doi: 10.5650/jos.ess14058. [DOI] [PubMed] [Google Scholar]
- 13.Saikia J.P., Bharali P., Konwer B.K. Possible protection of silver nanoparticles against salt by using rhamnolipid. Colloids Surf. B Biointerfaces. 2013;104:330–332. doi: 10.1016/j.colsurfb.2012.10.069. [DOI] [PubMed] [Google Scholar]
- 14.Leney A.C., Darestani R.R., Li J., Nikjah S., Kitova E.N., Zou C., Cairo C.W., Xiong Z.J., Privé G.G., Klassen J.S. Picodiscs for facile protein-glycolipid interaction analysis. Anal. Chem. 2015;87:4402–4408. doi: 10.1021/acs.analchem.5b00170. [DOI] [PubMed] [Google Scholar]
- 15.Roy B., Mukhopadhyay B. Sulfuric acid immobilized on silica; an excellent catalyst for Fischer type glycosylation. Tetrahedron Lett. 2007;48:3783–3787. [Google Scholar]
- 16.Colombo D., Ronchetti F., Scala A., Toma L. Bioactive glycoglycerolipid analogues: an expeditious enzymatic approach to mono- and diesters of 2-O-β-D-galactosylglycerol. Tetrahedron Asymmetry. 1998;9:2113–2119. [Google Scholar]
- 17.Jiang L., Shen C., Long X., Zhang L., Meng Q. Rhamnolipids elicit the same cytotoxic sensitivity between cancer cell and normal cell by reducing surface tension of culture medium. Appl. Microbiol. Biotechnol. 2014;98:10187–10196. doi: 10.1007/s00253-014-6065-0. [DOI] [PubMed] [Google Scholar]
- 18.Lorent J.H., Quetin-Leclercqb J., Mingeot-Leclercq M.P. The amphiphilic nature of saponins and their effects on artificial and biological membranes and potential consequences for red blood and cancer cells. Org. Biomol. Chem. 2014;12:8803–8822. doi: 10.1039/c4ob01652a. [DOI] [PubMed] [Google Scholar]