Abstract
Sepsis is a life-threatening organ dysfunction caused by dysregulated host response to infection and characterized by redox imbalance and severe oxidative stress. Glutathione (GSH) serves several vital functions, including scavenging free radicals and maintaining intracellular redox balance. Extracellular GSH is unable to be taken into the majority of human cells, and the GSH prodrug N-acetyl-l-cysteine (NAC) does not exhibit promising clinical effects. γ-glutamylcysteine (γ-GC), an intermediate dipeptide of the GSH-synthesis pathway and harboring anti-inflammatory properties, represents a relatively unexplored option for sepsis treatment. The anti-inflammatory efficiency of γ-GC and the associated molecular mechanism need to be explored. In vivo investigation showed that γ-GC reduced sepsis lethality and attenuated systemic inflammatory responses in mice, as well as inhibited lipopolysaccharide (LPS)-stimulated production of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), high-mobility group box 1 (HMGB1), and nitric oxide (NO) and the expression of inducible NO synthase and cyclooxygenase 2 in RAW264.7 cells. Moreover, both in vivo and in vitro experiments demonstrated that γ-GC exhibited better therapeutic effects against inflammation compared with N-acetyl-L-cysteine (NAC) and GSH. Mechanistically, γ-GC suppressed LPS-induced reactive oxygen species accumulation and GSH depletion. Inflammatory stimuli, such as LPS treatment, upregulated the expression of glutathione synthetase via activating nuclear factor-erythroid 2-related factor (Nrf2) and nuclear factor kappa B (NF-κB) pathways, thereby promoting synthesis of GSH from γ-GC. These findings suggested that γ-GC might represent a potential therapeutic agent for sepsis treatment.
Abbreviations: CHX, cycloheximide; CLP, cecal ligation and puncture; COX-2, prostaglandin H2 synthase; GCL, γ-glutamylcysteine ligase; GSH, glutathione; GSS, glutathione synthetase; HMGB1, high mobility group box 1; IL-1β, interleukin-1 beta; iNOS, inducible nitric oxide synthases; LPS, Lipopolysaccharide; NAC, N-acetyl-L-cysteine; NF-κB, nuclear factor-kappa B; NO, nitric oxide; Nrf2, nuclear factor-erythroid 2-related factor; ROS, reactive oxygen species; TNF-α, tumor necrosis factor-α; γ-GC, γ-glutamylcysteine; γ-GT, γ-glutamyltranspeptidase
Keywords: γ-glutamylcysteine, Sepsis, Glutathione, N-acetyl-L-cysteine, Glutathione synthetase
Graphical abstract
Highlights
-
•
γ-GC reduces sepsis lethality and attenuates inflammatory responses in BALB/c mice.
-
•
γ-GC suppresses LPS-induced inflammation, ROS accumulation, and GSH depletion.
-
•
Nrf2 and NF-κB pathways are essential for upregulating GSS level to promote GSH synthesis from γ-GC.
-
•
γ-GC is more effective in attenuation inflammation than NAC and GSH.
1. Introduction
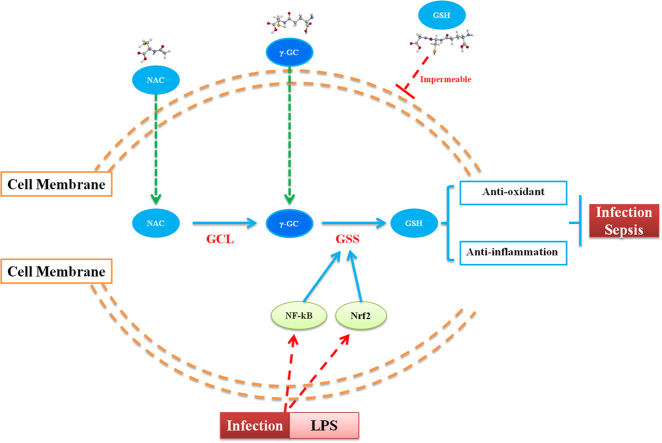
Sepsis is a major public health concern that shows high mortality and no effective approved clinical treatment. Dysregulation of the immuno-inflammatory response induced by pathogenic infection, injury, or xenobiotic infection can induce systemic inflammatory response syndrome and organ damage [1], [2]. Glutathione (GSH) is the most abundant non-protein thiol that defends against oxidative stress, but is depleted during the inflammatory response [3]. Additionally, GSH is a key determinant of redox signaling, vital in detoxification of xenobiotics, and regulates cell proliferation, apoptosis, immune function, and fibrogenesis [4], [5], [6], [7], [8], [9]. De novo synthesis of GSH occurs in the cytosol and involves two consecutive ATP-mediated enzymatic actions [10]. The first and rate-limiting step involves the formation of γ-glutamylcysteine (γ-GC) from glutamate and cysteine and catalyzed by γ-glutamylcysteine ligase (GCL), and the second step is catalyzed by glutathione synthetase (GSS) and involves condensation of γ-GC with glycine to produce GSH [11]. Based on the essential functions of GSH, elevation of GSH concentration in cells under various types of stress is important for protecting cells from injury.
Uptake of extracellular GSH is impossible in a majority of human cells due to the absence of a membrane-bound ectoenzyme γ-glutamyltranspeptidase (γ-GT) or an unfavorable concentration gradient across the cell membrane [11], [12]. Despite the use of N-acetyl-l-cysteine (NAC) to treat sepsis both directly as a GSH substitute and indirectly as a GSH precursor, clinical outcomes are controversial [13]. γ-GC, as an immediate precursor of GSH that differs from GSH in the absence of thermodynamic limitations regarding transmembrane transport, can be readily taken up by many cell types to promote GSH synthesis [14], [15], [16].
γ-GC exhibits antioxidant properties due to its cysteine residue. In infant mammals, γ-GC is initially provided in breast milk, as it cannot by produced by infants in sufficiently high enough quantities. Upon weaning, the infants are capable of producing γ-GC internally from dietary protein sources [17]. γ-GC is also present in some foods, including green beans, spinach, spices, mustard, and fenugreek [18]. Early mouse studies demonstrated that intraperitoneal administration of γ-GC restored depleted GSH content in organs [11], and that γ-GC displayed hepatoprotective effects against iron-overload-induced liver injury [19]. Le et al. [14] reported that γ-GC ameliorates oxidative injury in neurons and astrocytes in vitro and increases brain GSH levels in vivo [15]. Although γ-GC suppresses oxidative injury, its anti-inflammatory effects and related molecular mechanisms remain elusive.
Here, we showed that γ-GC improved the survival of septic mice induced by intraperitoneal injection of lipopolysaccharide (LPS) or cecal ligation and puncture (CLP) and suppressed the production of inflammatory mediators induced by LPS stimulation. Moreover, we compared the anti-inflammatory and anti-oxidative effects of γ-GC with NAC and GSH, as well as analyzed the mechanism associated with the anti-inflammatory effect of γ-GC.
2. Materials and methods
2.1. Reagents and antibodies
Gamma-glutamylcysteine (γ-GC, CAS No. 636-58-8) was obtained from Biospecialties International (Mayfield, Australia), as a sodium salt. Lipopolysaccharides (LPS, from Escherichia coli 0111: B4), N-acetyl-L-cysteine (NAC), 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), L-Glutathione reduced (GSH), cycloheximide (CHX) were purchased from Sigma Aldrich (St. Louis, USA). The monoclonal antibody to COX-2, iNOS, Nrf2, p-IκBα (Ser 32), NF-κB p65, NF-κB p-p65 (Ser 536), GCL catalytic subunit (GCLC), GCL modifier subunit (GCLM) were purchased from Santa Cruz Biotechnology (Dallas, USA). The monoclonal antibody to p-IKKα/β (Ser 176/180), IKKβ, HO-1, IκBα were purchased from Cell Signaling Technology (Beverly, USA). The polyclonal antibody to GSS was purchased from Proteintech Group (Rosemont, USA). The polyclonal antibody to GAPDH, LaminB, anti-rabbit-HRP IgG and anti-mouse-HRP IgG were purchased from Bioworld Technology (Minneapolis, USA).
2.2. Mouse inflammation models
BALB/c mice (male, 6 weeks old, 20 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. The animal study was approved by Medical Laboratory Animal Research Institute of Medical Sciences China [Permit Number: SYXK (Jing) 2014-0004]. All treatments of mice in this study were in strict agreement with guidelines on ARRIVE and recommendations from an NIH sponsored workshop regarding experimental design and reporting standards [20]. Mice were intraperitoneally (i.p.) injected with single dose of LPS (37.5 mg/kg) to produce endotoxin shock or challenged with CLP as previously described for polymicrobial sepsis [21], [22], [23]. Briefly, mice were anesthetized with sodium pentobarbital (30 mg/kg), and the severity of sepsis was highly rested with the extent of cecal ligation. The sham-operation which included the same procedure except for ligation and perforation of the cecum was conducted on control mice. Thirty minutes after each challenge, mice were oral-administrated with saline, γ-GC, NAC or GSH, respectively. The survival rates were monitored continuously for 4 days (n = 20 mice in each group). Serum TNF-α and IL-1β were measured at the indicated time after challenges.
2.3. Histological analysis
Mice were anaesthetized at 12 h after LPS or CLP challenges. Mouse lungs and livers were immersed in 4% paraformaldehyde for 24 h at 4 °C, and then were embedded in paraffin and sliced into five-micrometer sections. After staining with hematoxylin and eosin (H&E), sections were observed through light microscopy.
2.4. Cell culture
Murine macrophage-like RAW264.7 cells were obtained from the cell bank of the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Dulbecco's modification of Eagle's medium (Wisent, Montreal, Canada) supplemented with 10% (v/v) fetal bovine serum (Gibco, Carlsbad, USA) and with no antibiotics, and were maintained in an atmosphere of humidified 5% CO2 at 37 °C.
2.5. In vitro inflammatory mediator detection
RAW264.7 cells, seeded into 12-well plate 12 h before the experiment, were stimulated with or without LPS (1 μg/mL) for 24 h after treatment with γ-GC at different concentrations. Supernatants were collected and centrifuged at 10,000 rpm, 4 °C for 10 min. The cytokine concentrations were measured by using the quantitative ELISA kits according to the manufacturer's instructions. TNF-a and IL-6 ELISA kits were purchased from MultiSciences (Hangzhou, China) and HMGB1 ELISA kit was obtained from Shanghai Westang Bio-Tech Co., Ltd. The amount of nitrite was determined by using NO Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions.
2.6. Measurement of ROS production
RAW264.7 cells were seeded into 96-well plate 12 h before the experiment. After treatment, cells were washed twice with PBS and then incubated with 10 μM DCFH-DA probe (Beyotime, Shanghai, China) for 25 min. Cells were then rinsed twice with PBS and fluorescence assays were conducted with a fluorescence microplate reader (BIO-TEK, INC) at excitation/emission 488/525 nm [24].
2.7. Measurement of intracellular GSH levels
RAW264.7 cells were seeded into 12-well plate (1 × 105 cells/well) 12 h before the experiment. After treatment, cells were collected, washed with PBS and freeze-thaw breakage with liquid nitrogen and 37 ℃ water bath. Intracellular total GSH amount and oxidized GSH amount were determination using GSH assay kit (Beyotime) or GSH fluorometric assay kit (BioVision) according to the manufacturer's instructions.
2.8. Nuclear and cytosolic extract preparation
Nuclear and cytosolic fractions were separated by using NE-PER nuclear and cytoplasmic extraction Kit (Thermo Scientific, Rockford, IL, USA) according to the instructions specified by the manufacturer. Briefly, cells were harvested by centrifuging at 500 g for 5 min, followed by washing with PBS and then lysed in ice-cold cytoplasm extraction reagent I (CER I) for 10 min and ice-cold CER II for 1 min. Cytoplasmic component was extracted by centrifuging at 16,000 g for 5 min. Proteins of the nuclear material were then extracted by adding nuclear extraction reagent to the insoluble fraction with vigorous vortex per 10 min for four times and centrifugation at 16,000 g.
2.9. Western blotting
Total proteins were extracted from cells by using RIPA lysis and extraction buffer (Beyotime) containing protease inhibitor and phosphatase inhibitor (Roche, Rotkreuz, Switzerland). Equivalent amounts of protein were electrophoresed on 10% SDS-PAGE, and blotted onto polyvinylidene fluoride membranes (Millipore, Billerica, USA). After blocking with 5% non-fat milk solution for 1 h at room temperature, the membranes were washed with TBS-Tween-20 (0.1%, v/v) and then incubated with primary antibody at 4 °C overnight followed by incubating with HRP-conjugated secondary antibody for 1 h at room temperature. The antibody-antigen complexes were visualized by chemiluminescence method using the enhanced ECL immunoblotting system (Tanon, Shanghai, China) [25].
2.10. RNA extraction and quantitative PCR
Total RNA was extracted with Trizol reagent (Ambion, Carlsbad, USA). RNA was used to generate cDNA using PrimeScript reverse transcription reagents (TaKaRa, Shiga, Japan) according to manufacturer's protocol. 500 ng cDNA was amplified using 2 ×Tap plus master mix (Dye) (Vazyme Biotech, Nanjing, China). The mouse GSS transcript was amplified using the following primers: forward primer, 5′-GCCCCATTCACGCTCTTCCCC-3′, reverse primer, 5′-ATGCCCGGCCTGCT- TAGCTC-3′ and mouse GAPDH transcript used as an internal control was amplified using the following primers: forward primer, 5′-GAGAGTGTTTCCTCGTCCC- GTAG-3′, reverse primer, 5′-GCCTCACCCCATTTGATGTTAGT-3′. The PCR products were visualized in 1.0% agarose gels stained with ethidium bromide.
2.11. Statistical analysis
The experimental data were presented as mean ± SD from three times replicate experiments. The statistical analysis was performed using GraphPad Prism5 software. Data were analyzed by using analysis of variance (ANOVA). Statistical significance was determined using unpaired Student's two-tailed t-test for two data sets. The survival rates were constructed using the Kaplan-Meier curve, and differences in mortality were compared using the log-rank (Mantel-Cox) test. Statistical significance was defined as *, p < 0.05; **, p < 0.01; ***, p < 0.001,
3. Results
3.1. γ-GC reduces sepsis lethality and attenuates systemic inflammatory responses
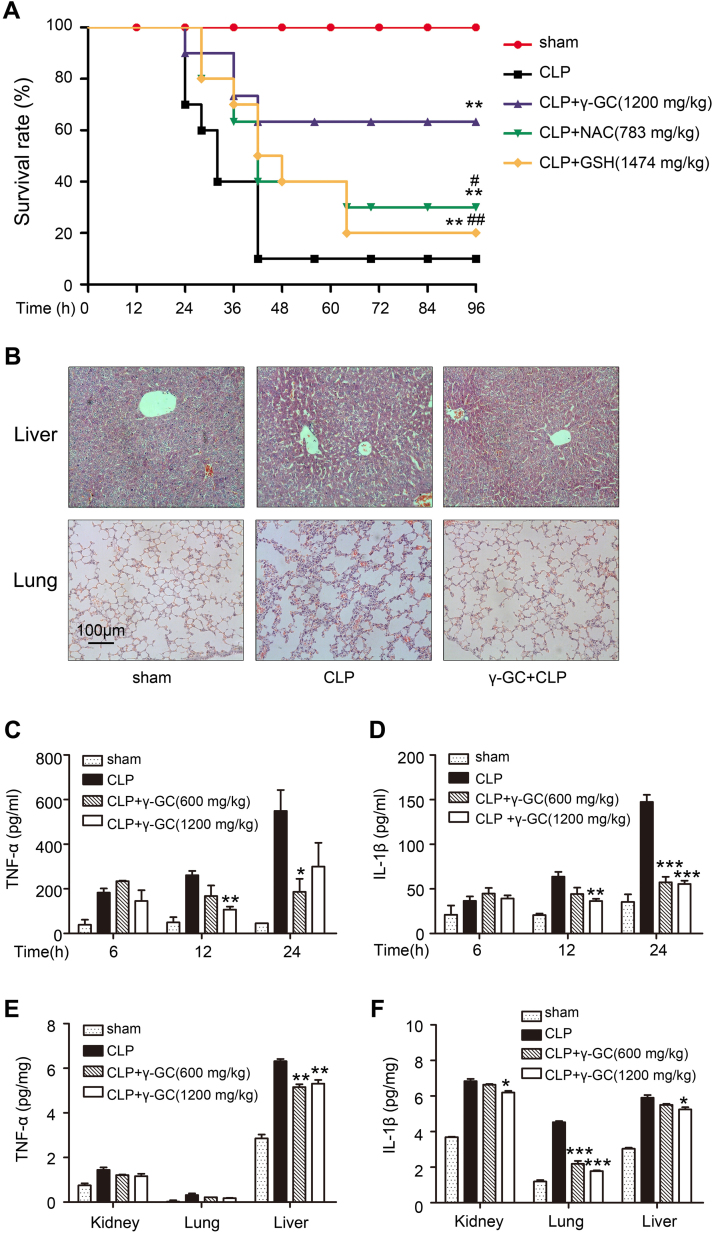
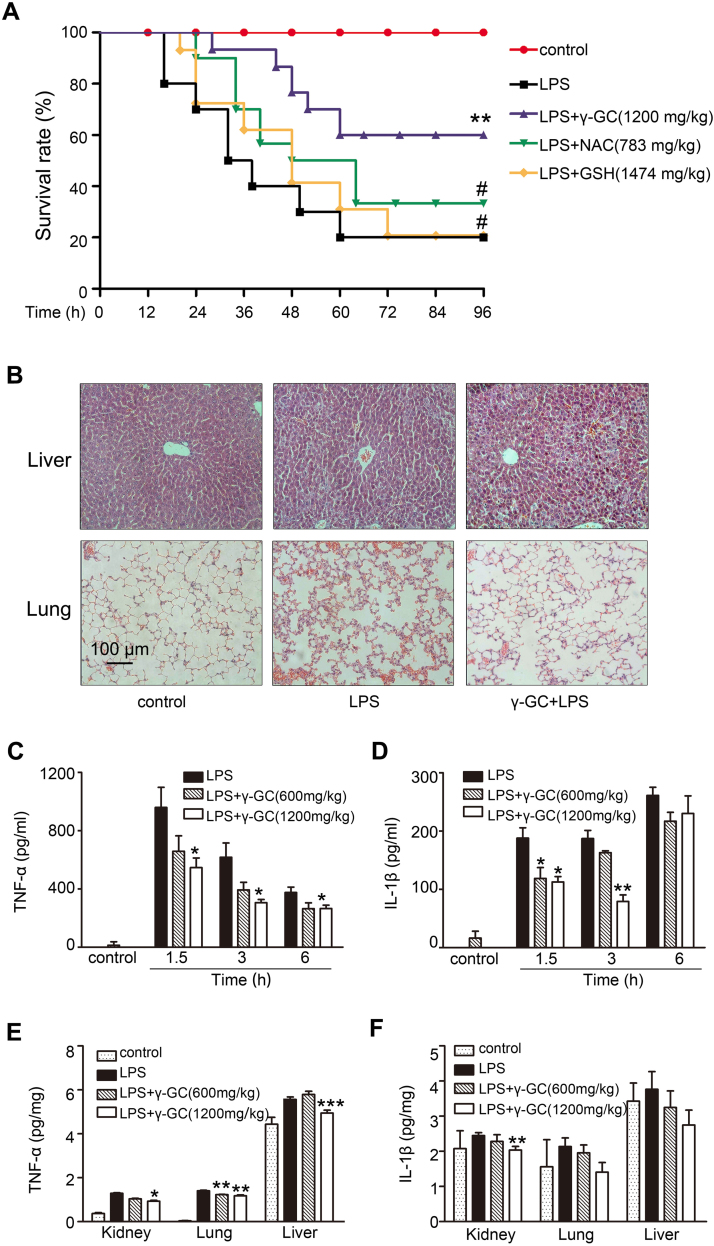
Sepsis mouse models were established by CLP or intraperitoneal injection of LPS. The CLP model is a well-established polymicrobial sepsis model demonstrated as the most representative animal model of human sepsis [26]. Mice injected with high-dose LPS suffer lethal endotoxin shock [27]. In the present study, oral administration of γ-GC (1200 mg/kg) following CLP surgery reduced the mortality rate from 90% to 40% (Fig. 1A), whereas γ-GC administration (1200 mg/kg) at 30 min post-LPS treatment reduced the mortality rate from 80% to 40% (Fig. 2A). Compared with the effects of NAC and GSH on the mouse mortality rate, γ-GC treatment was more effective in either CLP or LPS injection models (Figs. 1A and 2A). Additionally, we found that the release of the proinflammatory cytokines, TNF-α and IL-1β, induced by CLP or LPS in both serum and tissues attenuated following γ-GC administration (Figs. 1C–F and 2C–F).
Fig. 1.
γ-GC reduces CLP-induced sepsis lethality and attenuates systematic inflammatory responses. (A) Mice (n = 20/group) were treated with the indicated dose of γ-GC, NAC or GSH 30 min after CLP surgery. The survival rates of mice were monitored for 96 h continuously. (B) Lung and liver tissues of the mice were collected and subjected to Hematoxylin and Eosin staining 12 h after CLP, and examined by light microscopy (magnification, × 200). (C, D) Serum TNF-α and IL-1β levels were measured by ELISA at the indicated intervals after CLP surgery. (E, F) TNF-α and IL-1β in mouse liver, lung and kidney were measured by ELISA 24 h after CLP. *P < 0.05, ** P < 0.01, and ***P < 0.001 compared with CLP group; #P < 0.05 and ##P < 0.01 compared with CLP + γ-GC group. Data represent the mean ± SD.
Fig. 2.
γ-GC reduces lethality of endotoxic shock and attenuates systemic inflammatory responses. (A) Mice (n = 20/group) were treated with the indicated dose of γ-GC, NAC or GSH 30 min after LPS injection(37.5 mg/kg,ip). The survival rates of mice were monitored for 96 h continuously. (B) Lung and liver tissues of the mice were collected and subjected to Hematoxylin and Eosin staining 12 h after LPS treatment, and examined by light microscopy (magnification, × 200). (C, D) Serum TNF-α and IL-1β levels were measured by ELISA at the indicated intervals after LPS administration. (E) TNF-α level in mouse liver, lung and kidney were measured by ELISA 1.5 h after LPS administration. (F) IL-1β level in mouse liver, lung and kidney were measured by ELISA 6 h after LPS administration. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with LPS; #P < 0.05 and ##P < 0.01 compared with LPS + γ-GC. Data represent the mean ± SD.
Organ damage is a leading cause of death in patients with lethal inflammation. In sepsis, lung tissue is particularly susceptible to acute injury. Moreover, the liver is an important metabolic organ that exhibits antioxidant functions and in which γ-GC is converted to GSH. Therefore, we performed histological observation to lung and liver tissue. As shown in Figs. 1B and 2B, γ-GC administration alleviated mouse lung injury including alveolar wall thickening, alveolar destruction, bleeding, and liver injury, and inflammatory cell infiltrates induced by LPS or CLP challenge. These data indicated that γ-GC was effective in preventing LPS- or CLP-induced systemic inflammatory response and organ damage suggesting its potential therapeutic effects on sepsis.
3.2. γ-GC downregulates LPS-induced production of inflammatory mediators in RAW264.7 cells
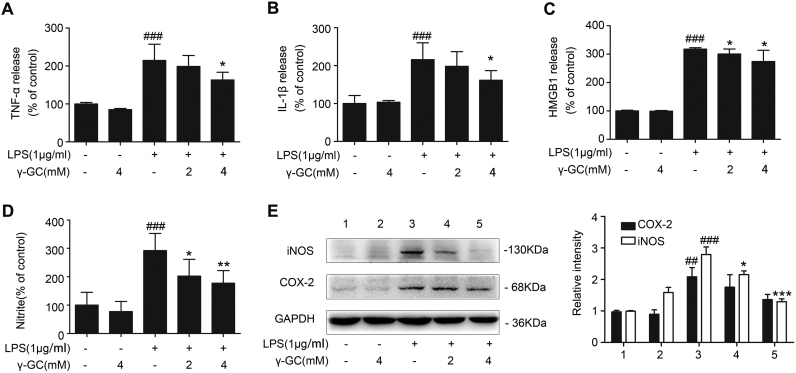
MTT detection showed that γ-GC from 20 μM to 12.5 mM did not significantly influence cell viability, suggesting its low cytotoxicity (Supplemental Fig. 1A). Release of inflammatory cytokines plays an essential role in inflammatory processes, we thus assess the effects of γ-GC on LPS-induced cytokine release from macrophages. RAW264.7 cells were incubated with various concentrations of γ-GC, and 24 h after LPS stimulation, secretion of TNF-α, IL-1β, and high-mobility group box 1 (HMGB1) in the culture medium was detected by ELISA. The results indicated that γ-GC administration attenuated the secretion of these LPS-induced proinflammatory cytokines (Fig. 3A–C).
Fig. 3.
γ-GC downregulates LPS-induced production of inflammatory mediators in RAW264.7 cells. RAW264.7 cells were pretreated with or without γ-GC (2 mM or 4 mM) for 10 min and stimulated with LPS for 24 h, and (A) TNF-α, (B) IL-1β, (C) HMGB1, and (D) NO levels in the culture supernatant were measured respectively using ELISA kits or Griess reagents. (E) iNOS and COX-2 levels in cell lysates were detected by western blot, with levels normalized against that of GAPDH. Values in control samples were arbitrarily set at 100%, with values in treated samples plotted as a percentage of this value. *P < 0.05, **P < 0.01, and ***P < 0.001 as compared with LPS; ##P < 0.01 and ###P < 0.001 as compared with control. Data represent the mean ± SD of three independent experiments. NS, not significant.
The two most prominent molecular mechanisms that mediate inflammatory processes are the formation of nitric oxide (NO) by inducible NO synthase (iNOS) and the production of prostaglandins by cyclooxygenase 2 (COX-2; prostaglandin H2 synthase) [28], [29]. We incubated RAW264.7 cells with various concentrations of γ-GC, and 24 h after LPS stimulation, western blot analysis was performed on cell lysates. We found that γ-GC attenuated LPS-elevated protein level of iNOS and COX-2 (Fig. 3D). Furthermore γ-GC reduced LPS-triggered NO release (Fig. 3E). These results indicated that γ-GC attenuated LPS-stimulated inflammatory responses of macrophages.
3.3. γ-GC protects cells against LPS-induced oxidative stress and elevates intracellular GSH level
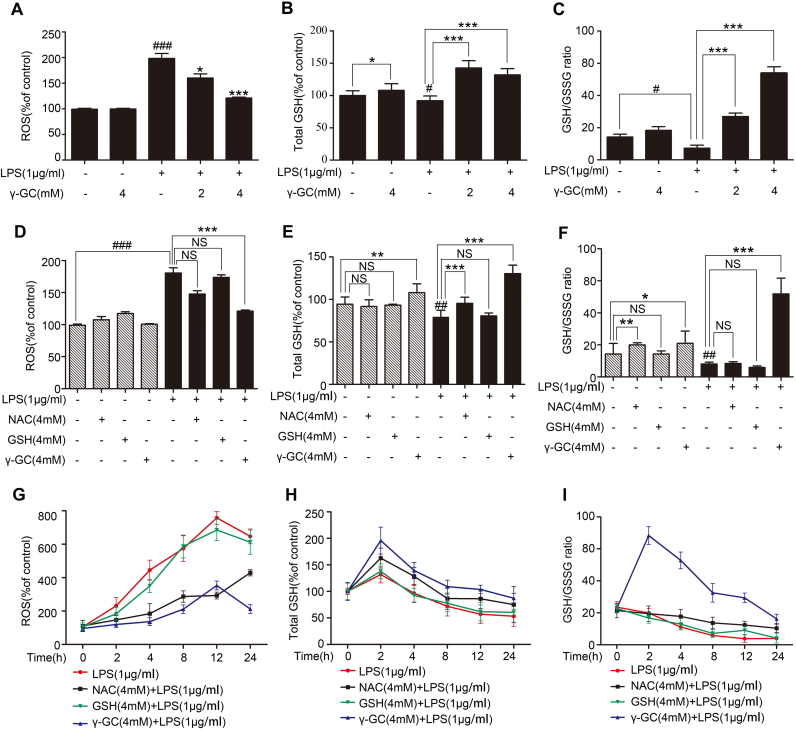
Reactive oxygen species (ROS) are generated in cellular response to xenobiotics, cytokines, and bacterial invasion. Oxidative stress refers to the imbalance due to excess ROS or oxidants over the capability of the cell to mount an effective antioxidant response [30]. We found that γ-GC administration decreased ROS levels induced by LPS in RAW264.7 cells (Fig. 4A). γ-GC is the immediate precursor to GSH, which is generated during cellular response to inflammatory stimuli [3]. As shown in Fig. 4B, the γ-GC treatment significantly elevated intracellular GSH level. Besides, 4 h after 2 μM or 4 μM γ-GC treatment, GSH/GSSG(oxidized GSH) ratio apparently increased, thereby alleviating LPS-induced decreases in GSH levels (Fig. 4C).
Fig. 4.
γ-GC alleviates LPS-induced oxidative stress. RAW264.7 cells were pretreated with or without γ-GC (2 mM or 4 mM) for 10 min and then stimulated with LPS (1 μg/mL) for 1 h. (A) Intracellular ROS were measured using a microplate reader after DCFH-DA staining. (B, C) Intracellular total GSH and reduced GSH levels were measured using an enzymatic recycling method. (D–F) RAW264.7 cells were pre-incubated with NAC, reduced GSH, or γ-GC at the same concentrations (4 mM) for 30 min and stimulated with LPS (1 μg/mL) for 1 h (##P < 0.01 and ###P < 0.001 compared with control; *P < 0.05 and **P < 0.01 compared with LPS stimulation). (G–I) RAW264.7 cells were pre-incubated with NAC, reduced GSH or γ-GC at the same concentrations (4 mM) for 30 min and stimulated with LPS (1 μg/mL) for the indicated time. Intracellular ROS, total GSH, and reduced GSH were measured. *P < 0.05, **P < 0.01, and ***P < 0.001, ##P < 0.01 and ###P < 0.001. Data represent the mean ± SD of three independent experiments. NS, not significant.
To further evaluate the effects of γ-GC on oxidative stress, we compared γ-GC with NAC and GSH during stress response. NAC is a source of cysteine used in the production of γ-GC, and GSH is the outcome of GSS-catalyzed transformation of γ-GC. Our results showed that γ-GC administration provided better protection against lethal shock as compared with NAC or reduced GSH (Figs. 1A and 2A). We thus compared the effects of γ-GC, NAC and GSH on cellular ROS, total GSH, and GSH/GSSG ratio levels following LPS stimulation to RAW264.7 cells. Results showed that γ-GC, at concentration of 4 μM, inhibited the elevation of ROS induced by LPS treatment for 1 h, whereas NAC and GSH did not significantly alter the ROS levels (Fig. 4D). As shown in Fig. 4G, cellular ROS level progressively increased during 12 h LPS treatment, which was suppressed by γ-GC and NAC but not GSH. The further study showed that γ-GC significantly increased intracellular GSH and GSH/GSSG ratio level 1 h after LPS stimulation and NAC only slightly increase GSH level but not affected GSH/GSSG ratio level (Fig. 4E and F). Two hours after LPS treatment intracellular GSH and GSH/GSSG ratio level declined gradually (Fig. 4H and I; Supplemental Fig. 1B), whereas, γ-GC significantly increased GSH and GSH/GSSG ratio level. Although NAC also raised GSH and GSH/GSSG ratio level after LPS treatment, its efficiency was much weaker than γ-GC. These results indicated that γ-GC exhibited more effective in raising cellular GSH level, especially the reduced GSH level, than NAC under LPS stimulation, suggesting that the stronger anti-inflammatory activity of γ-GC was related with its capacity to elevate cellular GSH level.
3.4. LPS promotes GSS expression in RAW264.7 cells through Nrf2 and NF-κB pathways
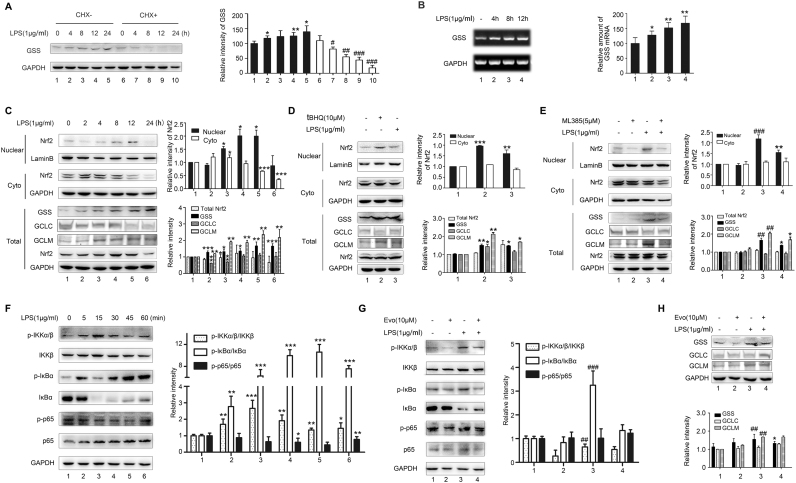
As shown in Fig. 4B and C, intracellular GSH levels were elevated following γ-GC treatment. Interestingly, the higher GSH level was observed in LPS-stimulated cells following γ-GC treatment than that in control cells. GSH synthesis from γ-GC and glycine is catalyzed by GSS [11], [31], we thus investigated the effect of LPS on GSS level in RAW264.7 cells. Cells were incubated with LPS (1 μg/mL) for various time periods in the presence or absence of the protein-synthesis inhibitor cycloheximide (CHX). Western blot analysis indicated that LPS stimulation increased the synthesis of GSS protein in a time-dependent manner (Fig. 5A). Subsequent quantitative polymerase chain reaction (qPCR) demonstrated that LPS increased in GSS mRNA level (Fig. 5B). These data indicated that LPS upregulated the expression of GSS protein at transcription and translation levels.
Fig. 5.
LPS elevates GSS expression in RAW264.7 cells through the Nrf2 and NF-κB pathways. (A) RAW264.7 cells were pretreated with or without CHX (1 μg/mL) for 30 min, followed by LPS stimulation (1 μg/mL) for the indicated time. GSS level in cell lysates were detected by western blot (*P < 0.05 compared with control, #P < 0.05, ##P < 0.01, and ###P < 0.001 compared with CHX + control). (B) RAW 264.7 cells were treated with LPS (1 μg/mL) for the indicated time, and total RNA was isolated and subjected to GSS mRNA level detection by quantitative PCR. (C) RAW 264.7 cells were treated with LPS (1 μg/mL) for the indicated time, and nuclear and cytoplasmic fractions were extracted for immunoblot analysis using antibodies against Nrf2, GSS, GCLC, and GCLM. (D) RAW 264.7 cells were pretreated with Nrf2 inducer tBHQ (10 μM) (*P < 0.05, **P < 0.01, and ***P < 0.001 compared with control) or (E) were pre-incubated with Nrf2 inhibitor ML385 (5 μM) for 30 min, and than were stimulated with LPS (1 μg/mL) for 12 h. Nuclear and cytoplasmic fractions were extracted for immunoblot analysis using antibodies against Nrf2, GSS, GCLC, and GCLM (##P < 0.01 and ###P < 0.001 compared with control; *P < 0.05 and **P < 0.01 compared with LPS stimulation). (F) RAW 264.7 cells were treated with LPS (1 μg/mL) for the indicated time and analyzed by western blot using the indicated antibodies (*P < 0.05, **P < 0.01, and ***P < 0.001 compared with control). (G) RAW264.7 cells were pre-incubated with an inhibitor of IκB kinase Evo (10 μM) for 30 min and stimulated with LPS (1 μg/mL) for 15 min. Cell lysates were detected by western blot through using indicated antibodies. (I) RAW264.7 cells were pre-incubated with Evo (10 μM) for 30 min and stimulated with LPS (1 μg/mL) for 12 h. Cell lysates were detected by western blot using antibodies against Nrf2, GSS, GCLC, and GCLM (*P < 0.05 compared with LPS stimulation; ##P < 0.01 compared with control). Data represent the mean ± SD of three independent experiments.
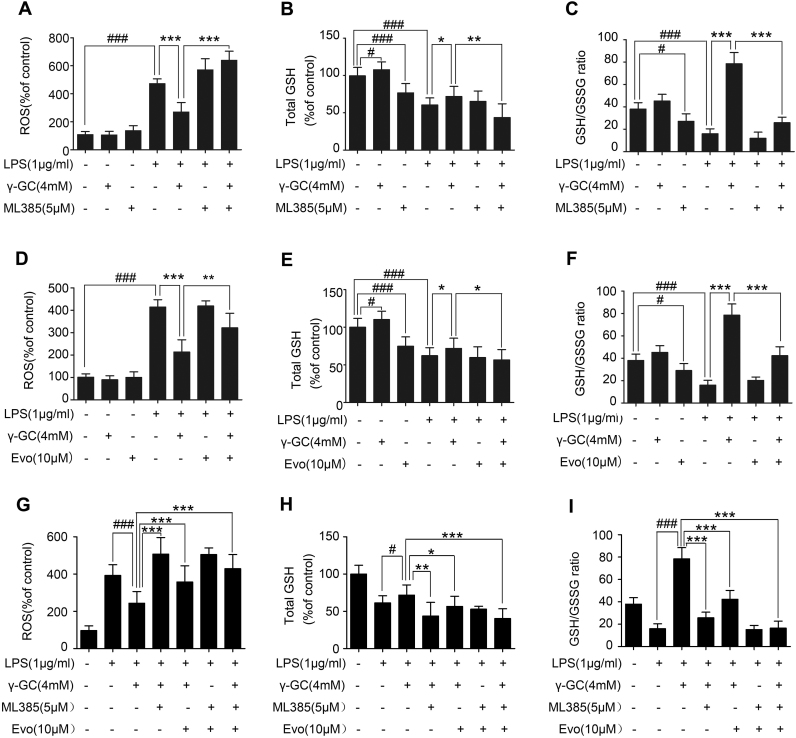
Nrf2 and NF-κB are transcription factors that play important roles in LPS-mediated inflammatory responses [32], [33]. To investigate the mechanisms by which LPS affects GSS expression, we evaluated activation of the Nrf2 and NF-κB pathways. Our results revealed that total Nrf2 level increased from 8 to 12 h after LPS treatment and reached to the highest level 12 h after LPS treatment (Fig. 5C). Nuclear Nrf2 also increased from 8 to 12 h after LPS treatment (Fig. 5C). Meanwhile, GSS and GCLM expression increased after 8 h treatment of LPS, whereas no alteration were observed in GCLC expression (Fig. 5C). Additionally, treatment of cells with a Nrf2-specific inducer tBHQ increased GSS and GCL protein level, in contrary, treatment with a Nrf2-specific inhibitor ML385 reduced GSS and GCLM level and had no effect on GCLC level (Fig. 5D and E). We then evaluated the activation of NF-κB pathway. LPS resulted in the phosphorylation of IKKα/β and IκBα in RAW 264.7 cells 5 min after challenge and peaked at 15 min (Fig. 5F). Subsequently, evodiamine (Evo), an inhibitor of IκB kinase, suppressed GSS expression but did not affected GCLM level (Fig. 5G). In further experiment, when we treated RAW 264.7 cells with ML385 and Evo to suppress the activation of both Nrf2 and NF-κB pathways, we found that γ-GC-mediated attenuation of ROS level and increase of total GSH and GSH/GSSG ratio level were prevented (Fig. 6). These results suggested that LPS promoted GSS expression by activating the Nrf2 and NF-κB pathways (Fig. 7).
Fig. 6.
Nrf2 and NF-κB pathways influence GSS expression. (A–C) RAW264.7 cells were pre-incubated with or without ML385 (5 μM) for 30 min or γ-GC (4 mM) and stimulated with LPS (1 μg/mL) for 12 h. (D–F) RAW264.7 cells were pre-incubated with or without Evo (5 μM) for 30 min or γ-GC (4 mM) and stimulated with LPS (1 μg/mL) for 12 h. (G–I) RAW264.7 cells were pre-incubated with or without Evo (5 μM) or Evo (5 μM) for 30 min or γ-GC (4 mM) and stimulated with LPS (1 μg/mL) for 12 h. Intracellular ROS levels, total GSH, and reduced GSH level were measured using the described methods. *P < 0.05, **P < 0.01, and ***P < 0.001; #P < 0.05 and ###P < 0.001. Data represent the mean ± SD of three independent experiments.
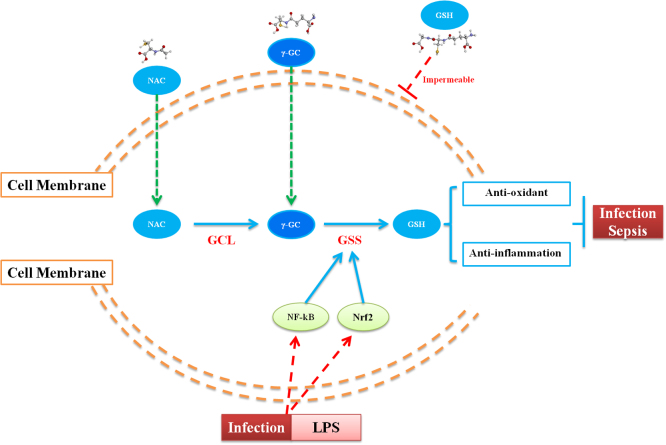
Fig. 7.
A proposed schematic diagram describing the protective mechanisms of γ-GC against LPS-induced sepsis.
4. Discussion
In the present study, we determined the efficacy of γ-GC in enhancing cellular GSH level and suppressing inflammation,and our findings suggested that γ-GC was potential to act as a therapeutic candidate for sepsis. Sepsis is a clinical condition associated with GSH depletion and a major public health concern [34], [35], [36], [37]. A series of therapies and adjunctive drugs, such as glucocorticoids, insulin, and recombinant activated protein C, have been used for clinical treatment for sepsis [38], [39], [40], however, these drugs induce several side effects ranging from potentially life-threatening (e.g., cardiovascular events and infections) to those less clinically serious (e.g., sleep disturbances, stomach upset during the treatment period) [41], [42], [43]. γ-GC is a relatively unexplored option for sepsis treatment, despite its exhibiting anti-inflammatory effects with little or no side effects [44].
In the present study, we investigated the anti-inflammatory potential of γ-GC and compared the anti-inflammatory and anti-oxidant activity of γ-GC with NAC and GSH. Our findings suggest γ-GC as having potential advantages over the other two substrates in the context of promoting anti-inflammation effects. GSH was limited for being a therapeutic agent because of its unfavorable biochemical and pharmacokinetic properties [45]. NAC is one kinds of source of thiol groups able to trigger GSH synthesis, and as a result, to promote detoxification and to act as a scavenger of ROS [46], [47], [48]. However, because of its low bioavailability, the results of NAC in animal models and in vitro experiments are controversial. Besides, the synthesis of GSH from NAC needs three enzymes and the effect of GCL is rate limited. Exogenous γ-GC would be directed into the cell and catalyzed by GSS to elevate intracellular GSH levels. Additionally, γ-GC does not result in allosteric feedback inhibition of GSS activity [31], which suggests that it is more effective for γ-GC in GSH synthesis compared with NAC. Altogether, our findings are consistent with these reports with.
As the precursor to GSH, γ-GC elevated GSH and GSH/GSSG ratio levels slightly in the absence of LPS, however, upon LPS stimulation, total GSH level as well as GSH/GSSG ratio in cells pretreated with γ-GC markedly increased. We observed time-dependent increases in GSS mRNA and protein levels following LPS stimulation, which subsequently promoted increases in GSH level. This could be explained by the LPS-induced inflammatory response stimulating the production and activation of enzymes associated with GSH biosynthesis [49]. Compared with other enzymes related to GSH biosynthesis, GSS was more sensitive to inflammation or LPS stimulation. GSS is the key enzyme that catalyzes γ-GC and glycine to produce GSH. It has been reported that decreased GGS activity alone in the absence of changes in GCL levels resulted in attenuated GSH level following surgical trauma in human skeletal muscle [50]. Additionally, Choi et al. described that the decreased hepatic GSH level was correlated with reduced GSS activity in Tat-transgenic mice [51]. Our findings together with previous studies suggested more importance for GSS in GSH synthesis in inflammatory and oxidant stresses.
It has been reported that bucillamine induces the increase of both γ-GCS and GSS expression by activating Nrf2 and thereby, further induces intracellular GSH increase [52], [53]. Additionally, NF-κB activates the rat GSS promoter, albeit indirectly via activator protein 1 [54]. To investigate the underlying mechanism involved in LPS-induced elevations in GSS levels, we assessed activation of the Nrf2 and NF-κB pathways. Assays involving inhibitors or inducers of these two signaling pathways revealed that Nrf2 and NF-κB activation was essential for LPS-induced GSS upregulation. A previous study indicated that activated Nrf2 signaling upregulates the transcription of glutathione reductase (GR), which increased GR activity and resulted in reduction of GSSG to GSH [55]. This could explain our observation of γ-GC administration resulting in elevated GSH/GSSG ratios upon LPS stimulation, as upregulated GSS levels promoted GSH synthesis, and upregulated GR levels likely promoted GSSG reduction to GSH.
In conclusion, our in vitro and in vivo results demonstrated the therapeutic potential of γ-GC for treating sepsis. We found that γ-GC administration protected mice from CLP- or LPS-induced lethal toxicity, and that the mechanism associated with this anti-inflammatory activity involved increased intracellular GSH levels and inhibition of ROS accumulation. Moreover, γ-GC exhibited better anti-inflammatory effects as compared with those observed following NAC administration, suggesting γ-GC as a potentially efficacious therapeutic reagent for sepsis treatment.
Acknowledgments
We thank Dr. Martin Zarka (Biospecialties International, Mayfield, NSW, Australia) for providing γ-GC sample. This work was supported by Grants from the National Natural Science Foundation of China (Nos. 31571166, 81602733 and 81711703).
Acknowledgments
Authors’ contributions
YY and LL: Procurement of funding, conception and design, acquisition of data, analysis and interpretation of data, writing of the manuscript. QH: Conception and design, interpretation of data, revision of the manuscript. XD: Analysis and interpretation of data. YF: Acquisition of data, analysis and interpretation of data, Statistical analysis. PC, ZY and LL: Procurement of funding, conception and design, study supervision, revision of the manuscript. All authors read and approved the final manuscript.
Conflict of interest statement
The authors have no conflict of interests to declare.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.09.019.
Contributor Information
Peng Cao, Email: caopeng@jsatcm.com.
Zhimin Yin, Email: yinzhimin@njnu.edu.cn.
Lan Luo, Email: lanluo@nju.edu.cn.
Appendix A. Supplementary material
Supplementary material
References
- 1.Macdonald J., Galley H.F., Webster N.R. Oxidative stress and gene expression in sepsis. Br. J. Anaesth. 2003;90:221–232. doi: 10.1093/bja/aeg034. [DOI] [PubMed] [Google Scholar]
- 2.Cawcutt K.A., Peters S.G. Severe sepsis and septic shock: clinical overview and update on management. Mayo Clin. Proc. 2014;89:1572–1578. doi: 10.1016/j.mayocp.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Song M., Kellum J.A., Kaldas H., Fink M.P. Evidence that glutathione depletion is a mechanism responsible for the anti-inflammatory effects of ethyl pyruvate in cultured lipopolysaccharide-stimulated RAW 264.7 cells. J. Pharmacol. Exp. Ther. 2004;308:307–316. doi: 10.1124/jpet.103.056622. [DOI] [PubMed] [Google Scholar]
- 4.DeLeve L.D., Kaplowitz N. Glutathione metabolism and its role in hepatotoxicity. Pharmacol. Ther. 1991;52:287–305. doi: 10.1016/0163-7258(91)90029-l. [DOI] [PubMed] [Google Scholar]
- 5.Ballatori N., Krance S.M., Notenboom S., Shi S., Tieu K., Hammond C.L. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 2009;390:191–214. doi: 10.1515/BC.2009.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nosareva O.L., Stepovaya E.A., Ryazantseva N.V., Shakhristova E.V., Egorova M.Y., Novitsky V.V. The role of the glutathione system in oxidative modification of proteins and dysregulation of apoptosis in Jurkat tumor cells. Bull. Exp. Biol. Med. 2017;164:199–202. doi: 10.1007/s10517-017-3957-x. [DOI] [PubMed] [Google Scholar]
- 7.Suthanthiran M., Anderson M.E., Sharma V.K., Meister A. Glutathione regulates activation-dependent DNA synthesis in highly purified normal human T lymphocytes stimulated via the CD2 and CD3 antigens. Proc. Natl. Acad. Sci. USA. 1990;87:3343–3347. doi: 10.1073/pnas.87.9.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pallardo F.V., Markovic J., Garcia J.L., Vina J. Role of nuclear glutathione as a key regulator of cell proliferation. Mol. Asp. Med. 2009;30:77–85. doi: 10.1016/j.mam.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Liu R.M., Gaston Pravia K.A. Oxidative stress and glutathione in TGF-beta-mediated fibrogenesis. Free Radic. Biol. Med. 2010;48:1–15. doi: 10.1016/j.freeradbiomed.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meister A., Anderson M.E. Glutathione. Annu. Rev. Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 11.Anderson M.E., Meister A. Transport and direct utilization of gamma-glutamylcyst(e)ine for glutathione synthesis. Proc. Natl. Acad. Sci. USA. 1983;80:707–711. doi: 10.1073/pnas.80.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witschi A., Reddy S., Stofer B., Lauterburg B.H. The systemic availability of oral glutathione. Eur. J. Clin. Pharmacol. 1992;43:667–669. doi: 10.1007/BF02284971. [DOI] [PubMed] [Google Scholar]
- 13.Ellouze O., Frikha N., Ouerghi S., Mestiri T., Salah Ben Ammar M. [N-acetylcysteine in septic shock] Tunis. Med. 2011;89:738–744. [PubMed] [Google Scholar]
- 14.Zarka M.H., Bridge W.J. Oral administration of gamma-glutamylcysteine increases intracellular glutathione levels above homeostasis in a randomised human trial pilot study. Redox Biol. 2017;11:631–636. doi: 10.1016/j.redox.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le T.M., Jiang H., Cunningham G.R., Magarik J.A., Barge W.S., Cato M.C., Farina M., Rocha J.B., Milatovic D., Lee E., Aschner M., Summar M.L. gamma-Glutamylcysteine ameliorates oxidative injury in neurons and astrocytes in vitro and increases brain glutathione in vivo. Neurotoxicology. 2011;32:518–525. doi: 10.1016/j.neuro.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson G., Bridge W. Glutamate cysteine ligase and the age-related decline in cellular glutathione: the therapeutic potential of gamma-glutamylcysteine. Arch. Biochem. Biophys. 2016;593:12–23. doi: 10.1016/j.abb.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Bounous G., Gold P. The biological activity of undenatured dietary whey proteins: role of glutathione. Clin. Investig. Med. 1991;14:296–309. [PubMed] [Google Scholar]
- 18.Demirkol O., Adams C., Ercal N. Biologically important thiols in various vegetables and fruits. J. Agric. Food Chem. 2004;52:8151–8154. doi: 10.1021/jf040266f. [DOI] [PubMed] [Google Scholar]
- 19.Salama S.A., Al-Harbi M.S., Abdel-Bakky M.S., Omar H.A. Glutamyl cysteine dipeptide suppresses ferritin expression and alleviates liver injury in iron-overload rat model. Biochimie. 2015;115:203–211. doi: 10.1016/j.biochi.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthr. Cartil. 2012;20:256–260. doi: 10.1016/j.joca.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y., Cao X., Yang Y., Wang J., Yang W., Ben P., Shen L., Cao P., Luo L., Yin Z. Glutathione S-transferase Pi prevents sepsis-related high mobility group box-1 protein translocation and release. Front. Immunol. 2018;9:268. doi: 10.3389/fimmu.2018.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L., Xie M., Yang M., Yu Y., Zhu S., Hou W., Kang R., Lotze M.T., Billiar T.R., Wang H., Cao L., Tang D. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat. Commun. 2014;5:4436. doi: 10.1038/ncomms5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan J.J., Jung J.S., Lee J.E., Lee J., Huh S.O., Kim H.S., Jung K.C., Cho J.Y., Nam J.S., Suh H.W., Kim Y.H., Song D.K. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat. Med. 2004;10:161–167. doi: 10.1038/nm989. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y., Luo L., Cai X., Fang Y., Wang J., Chen G., Yang J., Zhou Q., Sun X., Cheng X., Yan H., Lu W., Hu C., Cao P. Nrf2 inhibits oxaliplatin-induced peripheral neuropathy via protection of mitochondrial function. Free Radic. Biol. Med. 2018;120:13–24. doi: 10.1016/j.freeradbiomed.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y., Yin F., Hang Q., Dong X., Chen J., Li L., Cao P., Yin Z., Luo L. Regulation of endothelial permeability by glutathione S-transferase Pi against actin polymerization. Cell. Physiol. Biochem. 2018;45:406–418. doi: 10.1159/000486918. [DOI] [PubMed] [Google Scholar]
- 26.Rittirsch D., Huber-Lang M.S., Flierl M.A., Ward P.A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu Y., Xu X., Liu L., Mao S., Feng T., Lu Y., Cheng Y., Wang H., Zhao W., Tang W. Progranulin deficiency leads to severe inflammation, lung injury and cell death in a mouse model of endotoxic shock. J. Cell. Mol. Med. 2016;20:506–517. doi: 10.1111/jcmm.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 29.Turini M.E., DuBois R.N. Cyclooxygenase-2: a therapeutic target. Annu. Rev. Med. 2002;53:35–57. doi: 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- 30.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamazaki S., Muta T., Takeshige K. A novel IkappaB protein, IkappaB-zeta, induced by proinflammatory stimuli, negatively regulates nuclear factor-kappaB in the nuclei. J. Biol. Chem. 2001;276:27657–27662. doi: 10.1074/jbc.M103426200. [DOI] [PubMed] [Google Scholar]
- 33.Kensler T.W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 34.Hoetzenecker W., Echtenacher B., Guenova E., Hoetzenecker K., Woelbing F., Bruck J., Teske A., Valtcheva N., Fuchs K., Kneilling M., Park J.H., Kim K.H., Kim K.W., Hoffmann P., Krenn C., Hai T., Ghoreschi K., Biedermann T., Rocken M. ROS-induced ATF3 causes susceptibility to secondary infections during sepsis-associated immunosuppression. Nat. Med. 2011;18:128–134. doi: 10.1038/nm.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xing W., Yang L., Peng Y., Wang Q., Gao M., Yang M., Xiao X. Ginsenoside Rg3 attenuates sepsis-induced injury and mitochondrial dysfunction in liver via AMPK-mediated autophagy flux. Biosci. Rep. 2017;37 doi: 10.1042/BSR20170934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung H.Y., Kollmey A.S., Schrepper A., Kohl M., Blass M.F., Stehr S.N., Lupp A., Graler M.H., Claus R.A. Adjustment of dysregulated ceramide metabolism in a murine model of sepsis-induced cardiac dysfunction. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., Hotchkiss R.S., Levy M.M., Marshall J.C., Martin G.S., Opal S.M., Rubenfeld G.D., van der Poll T., Vincent J.L., Angus D.C. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volbeda M., Wetterslev J., Gluud C., Zijlstra J.G., van der Horst I.C., Keus F. Glucocorticosteroids for sepsis: systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2015;41:1220–1234. doi: 10.1007/s00134-015-3899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brunkhorst F.M., Engel C., Bloos F., Meier-Hellmann A., Ragaller M., Weiler N., Moerer O., Gruendling M., Oppert M., Grond S., Olthoff D., Jaschinski U., John S., Rossaint R., Welte T., Schaefer M., Kern P., Kuhnt E., Kiehntopf M., Hartog C., Natanson C., Loeffler M., Reinhart K. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N. Engl. J. Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 40.Bernard G.R., Vincent J.L., Laterre P.F., LaRosa S.P., Dhainaut J.F., Lopez-Rodriguez A., Steingrub J.S., Garber G.E., Helterbrand J.D., Ely E.W., Fisher C.J., Jr. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 41.Costello R., Patel R., Humphreys J., McBeth J., Dixon W.G. Patient perceptions of glucocorticoid side effects: a cross-sectional survey of users in an online health community. BMJ Open. 2017;7:e014603. doi: 10.1136/bmjopen-2016-014603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansen T.K., Thiel S., Wouters P.J., Christiansen J.S., Van den Berghe G. Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-binding lectin levels. J. Clin. Endocrinol. Metab. 2003;88:1082–1088. doi: 10.1210/jc.2002-021478. [DOI] [PubMed] [Google Scholar]
- 43.Gardlund B. Activated protein C (Xigris) treatment in sepsis: a drug in trouble. Acta Anaesthesiol. Scand. 2006;50:907–910. doi: 10.1111/j.1399-6576.2006.01086.x. [DOI] [PubMed] [Google Scholar]
- 44.Chandler S.D., Zarka M.H., Vinaya Babu S.N., Suhas Y.S., Raghunatha Reddy K.R., Bridge W.J. Safety assessment of gamma-glutamylcysteine sodium salt. Regul. Toxicol. Pharmacol. 2012;64:17–25. doi: 10.1016/j.yrtph.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Cacciatore I., Cornacchia C., Pinnen F., Mollica A., Di Stefano A. Prodrug approach for increasing cellular glutathione levels. Molecules. 2010;15:1242–1264. doi: 10.3390/molecules15031242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Millea P.J. N-acetylcysteine: multiple clinical applications. Am. Fam. Physician. 2009;80:265–269. [PubMed] [Google Scholar]
- 47.Bailey G.P., Wood D.M., Archer J.R., Rab E., Flanagan R.J., Dargan P.I. An assessment of the variation in the concentration of acetylcysteine in infusions for the treatment of paracetamol overdose. Br. J. Clin. Pharmacol. 2017;83:393–399. doi: 10.1111/bcp.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tobwala S., Khayyat A., Fan W., Ercal N. Comparative evaluation of N-acetylcysteine and N-acetylcysteineamide in acetaminophen-induced hepatotoxicity in human hepatoma HepaRG cells. Exp. Biol. Med. 2015;240:261–272. doi: 10.1177/1535370214549520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H., Liu H., Zhou L., Yuen J., Forman H.J. Temporal changes in glutathione biosynthesis during the lipopolysaccharide-induced inflammatory response of THP-1 macrophages. Free Radic. Biol. Med. 2017;113:304–310. doi: 10.1016/j.freeradbiomed.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo J.L., Hammarqvist F., Andersson K., Wernerman J. Surgical trauma decreases glutathione synthetic capacity in human skeletal muscle tissue. Am. J. Physiol. 1998;275:E359–E365. doi: 10.1152/ajpendo.1998.275.2.E359. [DOI] [PubMed] [Google Scholar]
- 51.Choi J., Liu R.M., Kundu R.K., Sangiorgi F., Wu W., Maxson R., Forman H.J. Molecular mechanism of decreased glutathione content in human immunodeficiency virus type 1 Tat-transgenic mice. J. Biol. Chem. 2000;275:3693–3698. doi: 10.1074/jbc.275.5.3693. [DOI] [PubMed] [Google Scholar]
- 52.Kim S.J., Ho Hur J., Park C., Kim H.J., Oh G.S., Lee J.N., Yoo S.J., Choe S.K., So H.S., Lim D.J., Moon S.K., Park R. Bucillamine prevents cisplatin-induced ototoxicity through induction of glutathione and antioxidant genes. Exp. Mol. Med. 2015;47:e142. doi: 10.1038/emm.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang H., Zeng Y., Lee T.D., Yang Y., Ou X., Chen L., Haque M., Rippe R., Lu S.C. Role of AP-1 in the coordinate induction of rat glutamate-cysteine ligase and glutathione synthetase by tert-butylhydroquinone. J. Biol. Chem. 2002;277:35232–35239. doi: 10.1074/jbc.M203812200. [DOI] [PubMed] [Google Scholar]
- 54.Morales A., Garcia-Ruiz C., Miranda M., Mari M., Colell A., Ardite E., Fernandez-Checa J.C. Tumor necrosis factor increases hepatocellular glutathione by transcriptional regulation of the heavy subunit chain of gamma-glutamylcysteine synthetase. J. Biol. Chem. 1997;272:30371–30379. doi: 10.1074/jbc.272.48.30371. [DOI] [PubMed] [Google Scholar]
- 55.Xu Y., Zhou X., Shi C., Wang J., Wu Z. alpha-Lipoic acid protects against the oxidative stress and cytotoxicity induced by cadmium in HepG2 cells through regenerating glutathione regulated by glutamate-cysteine ligase. Toxicol. Mech. Methods. 2015;25:596–603. doi: 10.3109/15376516.2015.1044150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material